Summary of Contents for MESI ABPI MD
- Page 1 Instructions for Use MESI ABPI MD Ankle-Brachial Index ISO 9001 Q-1664 ISO 13485 M-049...
- Page 2 DISTRIBUTOR INFORMATION CONTACT INFORMATION Address MESI, development of medical devices, Ltd. Leskoškova cesta 11a SI-1000 Ljubljana Slovenia, European Union Telephone +386 (0)1 620 34 87 E-mail info@mesimedical.com Website www.mesimedical.com...
- Page 3 Instructions for Use MESI ABPI MD Ankle-Brachial Index ISO 9001 Q-1664 ISO 13485 M-049...
-
Page 4: Table Of Contents
1.2.3 Safety measures 2 PRODUCT DESCRIPTION 2.1 WHAT IS IN THE PACKAGE 2.1.1 Accessories 2.2 INTENDED USE 3 TECHNICAL SPECIFICATIONS 3.1 MESI ABPI MD 3.1.1 Dimensions 3.1.2 Power & battery 3.1.3 Cuffs 3.2 CLASSIFICATION 3.3 OPERATING, TRANSPORTING AND STORAGE CONDITIONS 3.4 MEASUREMENT SPECIFICATIONS... - Page 5 5.3.1 Cuff placement 5.3.2 Performing the ABI measurement 5.4 REVIEWING AN ABI MEASUREMENT 5.4.1 Result screen 5.4.1.1 ABI values 5.4.1.2 Parameters 5.4.1.3 Waveforms 5.4.1.4 Comments 5.4.1.5 Adding patient 5.5 INTERPRETATION OF ABI RESULT 5.5.1 Detection of severe PAD and incompressible arteries 5.5.2 Pulse waveforms 5.5.3 Oscilation graph 6 MAINTENANCE...
-
Page 6: Safety And Legal Recommendations
SAFETY AND LEGAL RECOMMENDATIONS The MESI ABPI MD users must be adequately trained to use the device. Before the first use of the device, users must carefully read the entire instructions for use and follow the instructions for use of the connected equipment. -
Page 7: Product Description
PRODUCT DESCRIPTION The MESI ABPI MD (model name: ABPIMDD) is an automated device intended for use in the professional environment. It provides a fast, accurate and simple method for ABPI determina- tion. The measurement can be performed with a 2-step (using 4 PRODUCT cuffs –... -
Page 8: Accessories
ABI measurements of adult patients in the PAD risk group. The MESI ABPI MD is intended to be used solely in the profes- sional clinical environment by trained healthcare personnel who can correctly place blood pressure cuffs on the patient’s body, verify that these cuffs are inflating or deflating normally, and start... - Page 9 ABI. The device is recharged through the AC/DC power supply. The MESI ABPI MD device is not intended to be used during patient transport and in emergency medical services.
-
Page 10: Technical Specifications
TECHNICAL SPECIFICATIONS Listed below is the technical information regarding the MESI ABPI MD and its on-delivery specifications. TECHNICAL SPECIFICATI- 3.1 MESI ABPI MD 3.1.1 223 mm / 8.78 in Width DIMENSIONS Height 174 mm / 6.85 in Depth 86 mm / 3.38 in Weight 1000 g / 2.2 lbs... -
Page 11: Classification
TECHNICAL SPECIFICATIONS 3.2 CLASSIFICATION Protection against Class II electric shock Medical device Class IIa classification Applied parts (cuffs Type BF Applied part for arms and ankles) Software classification Class A RF emissions Group 1. Class A (CIPSR 11) 3.3 OPERATING, TRANSPORTING AND STORAGE CONDITIONS Operating Conditions: Temperature, 10°... -
Page 12: Measurement Specifications
TECHNICAL SPECIFICATIONS 3.4 MEASUREMENT SPECIFICATIONS Measurements using oscillometry and volume plethysmography: • Ankle-Brachial Pressure Index • Systolic blood pressure • Diastolic blood pressure • Mean average blood pressure • Heart rate Measurement range: • Pressure: 0 to 299 mmHg • Pulse rate: 30 to 199 beats per minute Accuracy: •... -
Page 13: Quick Measuring Guide
NOTE The MESI ABPI MD may be used on pregnant women. NOTE The MESI ABPI MD is not intended for use on new-borns or children under the age of 10 years. NOTE In case of the presence of intravenous cannulas or arteriovenous (AV) fistulas, the cuffs and measurement can cause injury to the limb. -
Page 14: Preparation For Measurement
QUICK MEASURING GUIDE 4.1 PREPARATION FOR MEASUREMENT MESI ABPI MD users must be adequately trained in use of the device. Must carefully read and follow the entire Instructions for Use prior to initial use of the device. 4.1.1 The operational environment of the device should be appropri- DEVICE ate to ensure accurate measurements. - Page 15 QUICK MEASURING GUIDE Step 1 Choosing the right colour Select the appropriate cuff, depending on the description and the colour of the cuff: COLOUR POSITION DESCRIPTION of the cuff on the cuff Right arm RIGHT ARM Left arm LEFT ARM YELLOW Right ankle RIGHT ANKLE...
-
Page 16: Performing An Abi Measurement
QUICK MEASURING GUIDE 4.1.4 PERFORMING AN ABI MEASUREMENT Step 1 Amputation option: If a patient has an amputated limb or is not compliant for ABI measurement due to severe and/or painful wounds, the ABI can be measured only on the patient’s existing limbs. Before the measurement, you can disable a selected cuff by clicking on the button and... -
Page 17: Performinga Blood Pressure Measurement
QUICK MEASURING GUIDE 4.1.5 PERFORMING A BLOOD PRESSURE MEASUREMENT Step 1 Step 2 Depending on the right (1) or left (2) arm, select Observe the position indication on the cuff. and place the correct cuff (red or yellow). Then press START and wait until the measure- ment is completed. -
Page 18: Results
QUICK MEASURING GUIDE 4.2 RESULTS Once the measurement is done, the system automatically switches to the result page. 4.2.1 On the top, the navigation menu provides the following actions: RESULTS OF ABI – Check the ABI results. For easier MEASUREMENT interpretation the results are colour-coded and a representative scale is given as a reference. -
Page 19: Results Of Blood Pressure Measurement
QUICK MEASURING GUIDE On the top, the navigation menu provides the following actions: 4.2.2 RESULTS OF – Check the blood pressure results. BLOOD PRESSURE For easier interpretation, the results MEASUREMENT are colour-coded and a representative scale is given for a reference. –... -
Page 20: Detailed Instructions
INSTRUCTIONS 5.1 FIRST TIME USE 5.1.1 The MESI ABPI MD package includes MESI ABPI MD device, set of colour-coded blood pressure cuffs (size M), AC/DC adaptor, BASIC FUNCTIONALITIES USB cable, instructions for use, calibration report and declaration of conformity. - Page 21 DETAILED INSTRUCTIONS The charging system works automatically. When the AC/DC adaptor is plugged in, the battery is charging and when charging, the battery charge indicator is animated: The device does not need to be switched on to charge the battery. When the charging process is completed, it terminates automatically.
-
Page 22: Changing The Device Settings
Hold the ON/OFF button for 10 seconds to hard reset the device. When using the MESI ABPI MD device for the first time, it is necessary to set the language, time and date. It is mandatory to set the exact... -
Page 23: Performing An Abi Measurement
The person carrying out the measurement should always remain by the patient’s side and closely monitor the measurement process. The MESI ABPI MD device comes with 4 cuffs for all limbs. The 5.3.1 ABI measurement can be operated with 4 or 3 cuffs. When using 4 CUFF PLACEMENT cuffs, the MESI ABPI MD uses the SmartArm™... - Page 24 DETAILED INSTRUCTIONS Option 1: ABI measurement with 4 cuffs Red cuff on Yellow cuff on right arm left arm Black cuff on Green cuff on right leg left leg Option 2.1: ABI measurement with 3 cuffs (right arm) Red cuff on right arm Black cuff on Green cuff on...
- Page 25 DETAILED INSTRUCTIONS Option 2.2: ABI measurement with 3 cuffs (left arm) Yellow cuff on left arm Green cuff on Black cuff on left leg right leg Place the appropriate cuff on the left/right arm and position the cuff ARM CUFF 1-2 cm above the elbow joint.
- Page 26 DETAILED INSTRUCTIONS Place the cuff so that there is two fingers’ width of room between the limb and the cuff. Make sure that you have chosen the correct size by referring to the SIZE marking and the OK area of the cuff. Place the appropriate cuff on the left/right leg and position the cuff ANKLE CUFF 2-3 cm above the ankle.
-
Page 27: Performing The Abi Measurement
DETAILED INSTRUCTIONS 5.3.2 PERFORMING AN ABI MEASUREMENT Step 1 Turn on the device by holding the ON/ OFF button for 2 seconds. Step 2 Amputation option: If a patient has an amputated limb or is not compliant for ABI measurement due to severe and/or painful wounds, the ABI can be measured only on patient’s existing limbs. - Page 28 DETAILED INSTRUCTIONS Step 4 During the measurement, the pressure waveforms show the pressure oscillation for each limb while the display next to the oscillation shows the current pressure in the corresponding cuff. Progress bar: The progress bar shows the timeline of the ABI measurement. When the bar comes to an end, the measurement of all blood pressure ends and all the cuffs deflate.
-
Page 29: Reviewing An Abi Measurement
DETAILED INSTRUCTIONS 5.4 REVIEWING AN ABI MEASUREMENT When the measurement process is completed, result will be 5.4.1 displayed on the screen. This consists of 5 different areas: ABI RESULT SCREEN values, Parameters, Waveforms, Comment, Add patient. NOTE If the results of the Ankle-Brachial Index measurement are very unusual, repeat the measurement three times. -
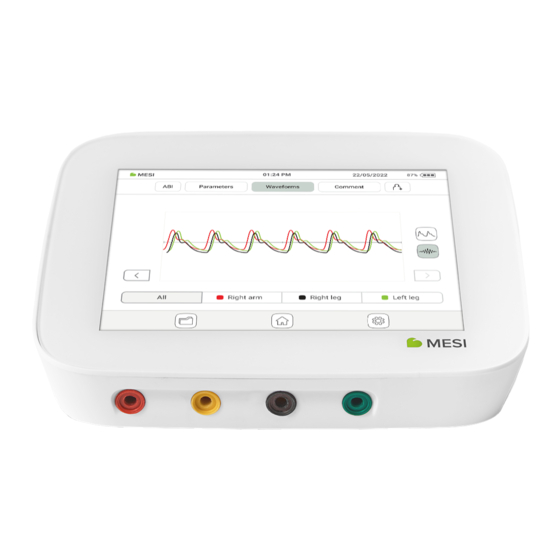
Page 30: Waveforms
DETAILED INSTRUCTIONS 5.4.1.3 In this section, a detailed view of the measured oscillation and pulse waveform graphs is provided. Waveforms 5.4.1.4 A comment can be added to every measurement. It will be stored and always available together with the recording report. Comments... -
Page 31: Adding Patient
DETAILED INSTRUCTIONS As an option, the device allows to assign the measurement to an 5.4.1.5 existing patient (1) or create a new patient’s profile (2). This option is not Adding patient required for a measurement to be performed. 5.5 INTERPRETATION OF AN ABI RESULT When the result ‘Abnormally weak pulse’... -
Page 32: Pulse Waveforms
DETAILED INSTRUCTIONS 5.5.2 The MESI ABPI MD uses the PADsense™ pattern recognition algorithm to automatically interpret the acquired pulse waveform PULSE and calculate the ABI with the result. However, to help the user WAVEFORM better understand the performed ABI measurement, this pulse waveform is available on the result screen. -
Page 33: Oscilation Graph
DETAILED INSTRUCTIONS 5.5.3 As with the pulse waveform, the MESI ABPI MD result page also OSCILATION displays oscillation graphs, which provide the user with pulse GRAPH waveform analysis throughout the ABI measurement. Below are a couple of examples of different oscillation graphs:... -
Page 34: Maintenance
• Use a soft lint-free cloth dampened with a proper cleaning agent to clean and disinfect the exterior of the MESI ABPI MD device. • Wipe off all residues of the cleaning solution with a dry cloth. -
Page 35: Disinfection
MAINTENANCE Cleaning – cuffs: • Clean the cuff surface by wiping it with a damp cloth with mild soap water or with wipes. • Do not wash the cuff or immerse it in water. Do not use petrol, thinners or similar solvents to clean the cuffs. Do not sterilise cuffs. -
Page 36: Product Life And Storage
Before using the device for the first time, read the instructions for use carefully and follow the recommendations. The MESI ABPI MD users must be adequately educated to use the device. The education must be performed by the trained MESI GENERAL representative. -
Page 37: Measurement Procedure
Only use the device when it is completely dry. The MESI ABPI MD is not intended for use in conjunction with high frequency surgical equipment. The AC/DC power supply must be connected to an easily accessible socket (the AC/DC power supply also serves for galvanic isolation). -
Page 38: Maintenance
GENERAL WARNINGS When repeating the Ankle-Brachial Pressure Index measurement or Blood Pressure measurement for several times a slight pain may appear at the measurement location. Other effects are excluded. The cables and accessories may negatively affect the EMC performance. The device while operated should not be stacked closer than cm from another medical device. -
Page 39: Functioning Of The Device
When moving the MESI ABPI MD stand, be sure to push the trolley and not the device. Never carry out repairs of any kind yourself. If a defect occurs, consult your dealer or distributor. -
Page 40: Errors
ERRORS ERRORS Solution Description Nr of Error ERROR Abnormally weak pulse The pulse was too Check the position of the cuff detected. Possibility of weak. Reposition and repeat the measurement. ERRORS severe PAD or incompress- and repeat the Follow Chapter 5.3.1 Cuff ible arteries. -
Page 41: Troubleshooting
TROUBLESHOOTING/ WARRANTY INFORMATION Unexpected result. Incorrect cuff placement. Reread the Instructions for Use and place the cuffs correctly. Patient moved during Repeat the measurement measurement. process. TROUBLE- SHOOTING Wrong cuff size used. Use cuffs of the correct size. Possible air leakage. Check the cuffs, the air tubes and the connectors, and replace them if necessary. -
Page 42: Standard Compliance
STANDARD COMPLIANCE The provisions of the Council Directive 93/42/EEC concerning medical devices were complied with. The standards in the table below were complied with. STANDARD Reference number Description COMPLIANCE (ID:year) EN 60601- Electrical medical equipment – Part 1: General 1:2006+A1:2013 requirements for basic safety and essential performance EN 60601-1-... -
Page 43: Manufacturer Declaration On Emc
3.1(b) of Directive 2014/53/EU 11.1 MANUFACTURER DECLARATION ON EMC MESI ABPI MD is intended for use in the electromagnetic environment specified below. The customer or the user of the above listed models should assure that they are used in such an environment. - Page 44 STANDARD COMPLIANCE Enclosure Port Immunity test levels Phenomenon Professional healthcare Home healthcare facility environment environment Electronic discharge ± 8 kV contact IEC 61000-4-2 ± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air Radiated RF EM 3 V/m f) 3 V/m f) fields a) 80 MHz –...
- Page 45 STANDARD COMPLIANCE All environments Phenomenon Test frequency Modulation Immunity test level (A/m) Rated power 30 A/m g) frequency magnetic fields d) e) 50 Hz or 60 Hz IEC 610004-8 30 kHz a) 134,2 kHz PM b) 65 c) 2,1 kHz 13,56 MHz PM b) 7,5 c)
- Page 46 STANDARD COMPLIANCE Input d.c. power port Immunity test levels Phenomenon Professional healthcare Home healthcare facility environment environment Electrical fast ± 2 kV transient/bursts 100 kHz Repetition frequency IEC 610004-4 (a, g) Surges (a, b, g) ± 0,5 kV, ± 1 kV line to line IEC 61000-4-5 ±...
- Page 47 STANDARD COMPLIANCE Signal input/output parts PORT Immunity test levels Phenomenon Professional healthcare Home healthcare facility environment environment Electrostatic ± 8 kV Contact discharge (ESD) ± 2, 4, 8, 15 kV Air IEC 61000-4-2 e) Electrical fast ± 1 kV transient/bursts 100 kHz Repetition frequency IEC 610004-4 b, f) 3 V (h)
-
Page 48: Essential Performance
ABI measurement under specified operating conditions. The measurement is defined as the measurement process on and data storage to the MESI ABPI MD device. Due its high sensitivi- ty, intended use and operating modes, the device is susceptible to EM interferences. If the essential performance of the device... -
Page 49: Important Labels
Do not dispose with domestic waste. IMPORTANT Observe the Operating Manual. LABELS Manufacturer: MESI, development of medical devices Ltd., Leskoškova cesta 11a, 1000 Ljubljana, Slovenia. CE mark. Indication of equipment that include RF transmitters. Consult Operating Manual. Operating Manual contains Warnings and cautions. - Page 50 IMPORATNT LABELS Trademark Green Dot. Refer to instruction manual. Date of manufacture. Temperature limit. Humidity limitation. Atmospheric pressure limiation. Directs in which way the box is to be placed to ensure it is upright. Fragile. Recyclable. Unique device identifier.
- Page 52 07-2022 / V. 2.0...






Need help?
Do you have a question about the ABPI MD and is the answer not in the manual?
Questions and answers