Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for NeuroMetrix DPNCheck NC-040
- Page 1 User Manual (Model: NC-040)
-
Page 2: Table Of Contents
Test Procedure ..........................12 Test Results Review – Single Limb Results .................. 16 Test Results Review – Results Available for Both Limbs ............. 18 Waveform Scaling ........................21 Recommended Testing Protocol ....................22 ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 3 APPENDIX C: SENSORY NERVE CONDUCTION PRINCIPLES & NERVE CONDUCTION TERMINOLOGY ........................29 APPENDIX D: UPLOAD TEST RESULTS TO COMPUTER ............30 APPENDIX E: TEMPERATURE COMPENSATION ..............32 APPENDIX F: TROUBLESHOOTING ..................33 APPENDIX G: ELECTROMAGNETIC COMPATIBILITY DECLARATION ........36 ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
-
Page 4: Chapter 1: Introduction
DPNCheck NC-040 Device User Manual INTRODUCTION CHAPTER 1: Indications For Use The NeuroMetrix DPNCheck® is intended to measure neuromuscular signals that are useful in diagnosing and evaluating systemic and entrapment neuropathies. Contraindications None. Warnings, Precautions & Safety Considerations • Federal law restricts this device to sale or use by or on the order of a health care provider appropriately licensed by the law in the state in which they practice •... -
Page 5: Device Overview
Infrared Thermometer – reads the patient’s skin surface temperature. • Stimulating Probes – non-invasively deliver electrical stimulation to the sural nerve. • Biosensor – a single patient-use biosensor is needed to conduct each test (NeuroMetrix model NC-DP2). • USB-C Port – for communication with a PC (optional). -
Page 6: Chapter 2: Setup
If any of the components appear to be damaged, or if the DPNCheck device fails to operate as described in this manual, contact NeuroMetrix immediately. Within the USA, customers should contact NeuroMetrix Customer Service: Phone: (888) 786-7287 Fax: (781) 663-3820 E-mail: Info@dpncheck.com... -
Page 7: Chapter 3: Dpncheck Operations Overview And Configuration
The flowchart below illustrates the basic steps for preparing a DPNCheck device and using the device to perform a test (including an optional step of uploading test results to a compatible PC application). ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 8: Battery Status
The following icons provide information on the biosensor connection status: No biosensor is connected. Biosensor is connected to the device. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 9: Test Mode Configuration And Device Information
Select the desired test mode by tapping “Bilateral” or “Unilateral” button on the screen. Unilateral Test Mode Selected Bilateral Test Mode Selected Once mode is selected, press the → to advance to the following Device Information screen: ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 10 Device Serial Number (SN): 15000001 (also viewable from the inside of the device battery compartment) • Hardware Version (HW) (example: A) • Software Version (FW) (example 1.0.77.29) Press CLOSE icon to exit this screen and return to Home screen. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
-
Page 11: Chapter 4: Operating Instructions
The device will power off automatically after 1-2 minutes of inactivity. Note: The last test is saved on the device until a new test is performed or until test is uploaded to the Reporter PC application. Contact NeuroMetrix Customer Service to obtain a copy of the Reporter application. -
Page 12: Patient Positioning
It is recommended that a sturdy chair with no wheels and a padded seat be used for patient comfort. Figure A: Preferred Position; side Figure B: Alternative Position; side Figure C: Alternative Position; prone Figure D: Alternative Position; Chair -- leg up ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
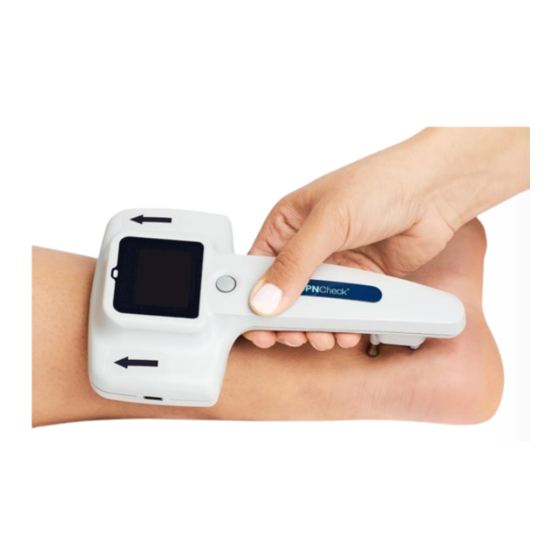
Page 13: Test Procedure
If you have not already done so, remove the protective liner from the foam and set aside for later use in protecting the foam adhesive between tests. Align the biosensor to the foam (provided with the biosensors). ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 14 Ready to Test screen (Bilateral Mode) (Unilateral Mode) Step 8: Apply a small amount of Signa conductive gel (provided with biosensors) to each probe. The head of the probe should be covered with gel. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
- Page 15 LED blinks green when each stimulus is delivered. Maintain firm pressure on both the biosensor and the probes throughout the test. Test times may vary per patient but normally lasts for 10-15 seconds. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
- Page 16 Limb Not Confirmed error. How to Stop the Test: Anytime during the test, you can lift the device from the patient to stop the test. See Appendix F for troubleshooting. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
-
Page 17: Test Results Review - Single Limb Results
If both limbs have a check mark above them, it indicates that results are available for both limbs (Bilateral Mode only). Please refer to Section 4.6 for instructions on how to switch results between two limbs. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 18 Amp/CV result screen → Waveform display for left limb Numerical results for left limb In certain cases, a test may only show an Amplitude result on numerical screen as seen below: ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
-
Page 19: Test Results Review - Results Available For Both Limbs
Press the filled blue square to view the test results of the other limb → Numerical results for right limb Numerical results for left limb ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 20 To toggle back to the numerical results screen, press the waveform area and it will highlight with a blue outline as seen below: Press the waveform area to switch to the Amp/CV result screen → Waveform display for left limb Numerical results for left limb ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
- Page 21 Make sure that the biosensor is NOT connected in order to view Test Results screen. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
-
Page 22: Waveform Scaling
When the Waveforms screen is first displayed, the waveform will be scaled to a fixed Y-axis of -20µV to +20µV. To change the scale, press the left side (scale) area of the screen and the waveform will toggle to an auto-scale view. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 23: Recommended Testing Protocol
Repeat use of the same biosensor on multiple patients will disable the device as indicated by the “Device Locked” error screen. Disabled devices must be returned to NeuroMetrix for a factory reset. Customers will be required to pay a service fee and all shipping and handling charges. -
Page 24: Chapter 5: Safety, Warranty Service, Care, And Service
Device or accessories. Spilling fluids may damage the device and/or present a fire or shock hazard. The DPNCheck device should only be used with NeuroMetrix biosensors. The DPNCheck device should only be placed over the intended anatomy as described in this manual. -
Page 25: Disposal Of Device, Batteries, And Biosensors
NeuroMetrix, Inc. This warranty does not cover any items that are in one or more of the following categories: software, external devices (except as specifically noted) or accessories or parts added to a NeuroMetrix device through authorized NeuroMetrix service centers. -
Page 26: Service
(freight prepaid) to addresses within the US or Canada. Shipments to other locations will be made freight collect. All parts removed from repaired products will become the property of NeuroMetrix, Inc. If NeuroMetrix repairs or replaces a product, the original warranty is not extended. - Page 27 DPNCheck NC-040 Device User Manual Devices Act (SMDA) for reporting to NeuroMetrix, and possibly the FDA, the occurrence of certain events. (FDA reportable events are detailed in 21 CFR Part 803.) In accordance with our Quality System, NeuroMetrix should be notified of any device failures or malfunctions.
-
Page 28: Appendix A: Specifications
Bipolar (2 cm separation) Conduction Velocity (CV) Onset of negative deflection (m/s) Response Amplitude Peak to peak (μV) Temperature Compensation Method Linear, 1.0 m/s per degree, maximum correction 5 m/s Reference Temperature ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 29: Appendix B: Symbols
Indicates that the transport package shall be kept away from rain and in dry conditions Do not re-use Use by Date ISO Symbol 7000-3082 for “Manufacturer” Manufacturer: NEUROMetrix, Inc. 4B Gill Street Woburn, MA 01801 ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 30: Appendix C: Sensory Nerve Conduction Principles & Nerve Conduction Terminology
DPNCheck NC-040 Device User Manual SENSORY NERVE CONDUCTION PRINCIPLES & NERVE APPENDIX C: CONDUCTION TERMINOLOGY ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 31: Appendix D: Upload Test Results To Computer
If the device is connected and powered on but Reporter is not open, the following message will display: 4. After connecting the device to the PC with the USB cable, the device will display the following screen sequence: ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 32 If using DPNCheck with Communicator the “Data Sent” screen will not display following upload. Obtain a Copy of the Reporter Application: Please contact NeuroMetrix Customer Service on how to obtain a copy of the Reporter Application: Phone: (888) 786-7287 Fax: (781) 663-3820 E-mail: info@dpncheck.com...
-
Page 33: Appendix E: Temperature Compensation
It is recommended in this case to warm the patient’s ankle to at least 24 C before repeating the test. Note: If there is noticeable damage to the infrared lens (located between the stimulating probes), the device should be repaired or replaced. ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... -
Page 34: Appendix F: Troubleshooting
When an error message is displayed with a symbol, pressing the screen will then display additional information regarding the error message. Error Message Help Screen (accessed by pressing the ? on the Status Error Message screen) ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A... - Page 35 DPNCheck NC-040 Device User Manual ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
- Page 36 DPNCheck NC-040 Device User Manual Error Message Help Screen (accessed by pressing the ? on the Status Error Message screen) ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...
-
Page 37: Appendix G: Electromagnetic Compatibility Declaration
Discharge Immunity ±16kV air (ESD); IEC 61000-4-2 Radiated RF; IEC 10 V/m; 80 10 V/m compliance level 61000-4-3 MHz to 2.5 GHz Magnetic Fields; 3 A/m 3 A/m compliance level IEC61000-4-8 ©2022 NeuroMetrix, Inc. All Rights Reserved. PN2205700 Rev A...





Need help?
Do you have a question about the DPNCheck NC-040 and is the answer not in the manual?
Questions and answers