Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for GSi ALLEGRO
- Page 1 HANDHELD SCREENING TYMPANOMETER ALLEGRO USER MANUAL...
- Page 2 Title: GSI Allegro Tympanometer User Manual Manufacturer Grason-Stadler 10395 West 70 Street Eden Prairie, MN 55344 Copyright © 2018 Grason-Stadler. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without the prior written permission of Grason-Stadler.
-
Page 3: Table Of Contents
GSI Allegro Tympanometer User Manual TABLE OF CONTENTS TABLE OF CONTENTS ............................... i Preface ................................... v Manual Conventions ................................v Regulatory Symbols ................................vi Device Symbols ................................. viii Important Safety Instructions ............................. ix Precautions ..................................ix Electromagnetic compatibility (EMC) considerations ......................x Warranty ..................................... - Page 4 GSI Allegro Tympanometer User Manual Handset ..................................... 7 Handset LED Indicators ............................... 8 Handset probe ..................................8 Printer ..................................... 8 Operation and Configuration ............................10 Start-up and menu displays .............................. 10 Main Menu Options ..............................10 Configuration ..................................11 Test sequence ................................13 Ear seal check................................
- Page 5 GSI Allegro Tympanometer User Manual Ear tips ................................... 16 Performing a test ................................17 Ear seal check ................................... 19 Error messages ................................. 23 Saving Results in the Database ............................. 24 Data entry ..................................24 Database full ..................................25 Sending the Results to a Printer ............................ 26 Printing results ..................................
- Page 6 GSI Allegro Tympanometer User Manual Appendix - EMC Guidance & Manufacturer’s Declaration ..................... 45 Electromagnetic Compatibility ............................45 Electrical Safety, EMC and Associated Standards ......................45 Appendix - Use with Non-medical Electrical Equipment....................51 D-0120695 Rev D 2022-06 Page iv...
-
Page 7: Preface
GSI Allegro Tympanometer User Manual REFACE This user manual provides information about the GSI Allegro tympanometer. This manual is intended for technically qualified personnel. Please note: This User Manual is not intended as a training manual for tympanometry. The reader should consult standard audiology texts for the theory and application of the screening tests provided by this instrument. -
Page 8: Regulatory Symbols
GSI Allegro Tympanometer User Manual EGULATORY YMBOLS Symbol Description Conforms to the Medical Device Regulation (EU) 2017/745. Symbol for "SERIAL NUMBER." GSI Part Number. Return to Authorized Representative, Special disposal required. Symbol for “European Authorized Representative.” Symbol for “Manufacturer.” Symbol for “Date of Manufacture.”... - Page 9 GSI Allegro Tympanometer User Manual Symbol Description Consult the operating instructions/directions for use. A copy of the operating manual is available on this website: www.grason-stadler.com A printed copy of the operating instructions can be ordered from Grason-Stadler for shipment within 7 days; or you can contact your local representative.
-
Page 10: Device Symbols
GSI Allegro Tympanometer User Manual EVICE YMBOLS The following symbols appear on the tympanometer, the instrument cradle or the mains adapter: Definition: Consult operating instructions. Definition: Type B applied part – an applied part providing protection against electric shock, particularly regarding allowable patient leakage current and patient auxiliary current. -
Page 11: Important Safety Instructions
AFETY NSTRUCTIONS WARNING The GSI Allegro instrument must be used only by medical professionals including, but not limited to, Physicians, Physician Assistants, Nurse Practitioners, Nurses, Audiologists and Medical Technologists knowledgeable in the theory and application of the screening tests provided by this instrument. It is intended for transient use as a screening and diagnostic tool;... -
Page 12: Electromagnetic Compatibility (Emc) Considerations
As with all instruments of this nature the measurements taken will be influenced by significant changes in elevation and pressure. The GSI Allegro tympanometer should be re-calibrated at the intended operating elevation. Do not attempt to open, modify or service the instrument. Return the instrument to the manufacturer or distributor for all repair and servicing requirements. -
Page 13: Warranty
GSI Allegro Tympanometer User Manual ARRANTY We, Grason-Stadler, warrant that this product is free from defects in material and workmanship and, when properly installed and used, will perform in accordance with applicable specifications. If within one year after original shipment, it is found not to meet this standard; it will be repaired, or at our option, replaced at no charge except for transportation costs, when returned to an authorized Grason-Stadler facility. -
Page 14: Introduction
NTENDED The GSI Allegro is intended to be used by an audiologist, ear nose and throat physician (ENT), hearing healthcare professional, or trained technician. The GSI Allegro is intended to be used in a hospital, clinic, or other healthcare facility with a suitable quiet testing environment such as a private exam room. -
Page 15: Contraindications
DMITTANCE MEASUREMENT The Allegro measures the admittance of the tympanic membrane and middle ear by playing a continuous 226Hz tone into the ear canal at a level calibrated to give 85dB SPL into a 2ml cavity. - Page 16 The Allegro can measure an acoustic reflex at any combination of 500Hz, 1000Hz, 2000Hz and 4000Hz. The maximum level for the reflex stimulus may be preset, along with the step size in dB between the three preceding lower levels of stimulus.
-
Page 17: Installation
XTERNAL NSPECTION Although this GSI Allegro was carefully tested, inspected, and packed for shipping, it is good practice after receiving the instrument to immediately examine the outside of the container for any signs of damage. Notify the carrier if any damage is observed. -
Page 18: Initial Set Up
OWER SUPPLY The GSI Allegro tympanometer is designed for continuous operation and is powered by a rechargeable Nickel-Metal Hydride (NiMH) battery-pack which is fitted in the instrument. If the instrument is placed onto its cradle the battery within it will be charged. -
Page 19: Cradle Led Indicators
For connected parts marked * only connect the parts or accessories supplied with the instrument or supplied by Grason-Stadler or a Grason-Stadler distributor. These parts have been tested for use with the GSI Allegro tympanometer for compliance with the standards IEC 60601-1 and IEC 60601-1-2. The use of accessories other than those specified may compromise compliance with these standards. -
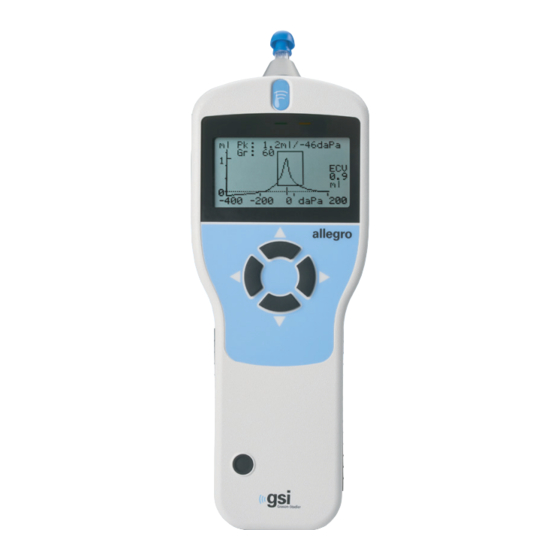
Page 20: Handset
Keys On/Off Press the On/Off key momentarily to turn the GSI Allegro on (refer to the diagram above). No warm-up time is required, although a short diagnostic routine will run for a few seconds. During this time the internal pump will operate. To switch off, again press and hold the On/Off key for a few seconds. -
Page 21: Handset Led Indicators
RINTER The GSI Allegro is supplied with portable thermal printer for printing tympanometric test results. Upon receipt of the printer it must be initially charged prior to use. Refer to the printer instructions for further details. Printing is from the cradle connected to the printer via the supplied serial cable. - Page 22 GSI Allegro Tympanometer User Manual D-0120695 Rev D 2022-06 Page 9...
-
Page 23: Operation And Configuration
PERATION AND ONFIGURATION Prior to performing tests with the GSI Allegro, the system should be properly configured. Set the values for the time and date to ensure that test data and calibration status are correctly identified. These values along with the instrument language and preferences for the parameters used in testing are set in the CONFIGURATION menu. -
Page 24: Configuration
GSI Allegro Tympanometer User Manual Press the down ▼ navigation keys to scroll through the menu until CONFIGURATION is highlighted and then press the right navigation key ► to select. ONFIGURATION The configuration menu contains 17 items with the values and defaults indicated in the table below. - Page 25 GSI Allegro Tympanometer User Manual Reflex Polarity Up or Down Down Reflex Filter 2 Hz or 1.5 Hz 2 Hz Reload Defaults Yes or No (Reflex Settings) Configuration Item Value Options Default Value (System Settings) Set Time/Date Date and time formatted selections Date currently set –...
-
Page 26: Test Sequence
By default, the reflex test at each frequency will stop at the lowest level of stimulus that produces a response. By setting REFLEX AUTO-STOP to NO the Allegro will test for a reflex at all selected D-0120695 Rev D 2022-06... -
Page 27: Reflex Polarity
OWER ELAY The GSI Allegro will switch off automatically if no key is pressed for a specified duration. Use the ▲ and ▼ keys to change this duration between 90 and 180 seconds and press the ► key to confirm and save the selection or the ◄ key to cancel. -
Page 28: Department
Press the ► key to confirm and save the selection or the ◄ key to cancel. ANGUAGE The GSI Allegro supports multiple languages. To set the operating language (English, German, French, Spanish, Portuguese or Italian) use the ▲ and ▼ keys to select the language. Press the ►... -
Page 29: Data Collection
GSI Allegro Tympanometer User Manual OLLECTION WARNING Ensure that the appropriate settings have been made before carrying out a test. See the information below and the CONFIGURATION options in the previous section. RIOR TO TESTING AND MBIENT CONDITIONS A qualified health care professional should perform a thorough otoscopic examination to establish that the condition of the ear is suitable for the test options selected and that no contraindications are present. -
Page 30: Performing A Test
GSI Allegro Tympanometer User Manual ERFORMING A TEST No specific action is required by the patient during the automatic test. However, the patient must be advised to remain still and avoid speaking or swallowing while the probe is applied to the ear. - Page 31 GSI Allegro Tympanometer User Manual TESTING LEFT EAR INSERT PROBE Cancel Place the ear tip into the ear canal to obtain a seal and the following messages will be displayed: TESTING LEFT EAR Equalizing Pressure Cancel TESTING LEFT EAR Pressure Settling...
-
Page 32: Ear Seal Check
GSI Allegro Tympanometer User Manual AR SEAL CHECK The type of ear seal check employed at the start of a test may be set in the CONFIGURATION menu. The default STANDARD option is adequate for most circumstances, and this checks that an adequate pressure can be created in the ear canal before starting the test. - Page 33 GSI Allegro Tympanometer User Manual Taking a tympanogram takes about 3 seconds. It is important not to move the probe and to ask the patient to remain very still during the test. When the tympanogram is complete the instrument will perform the reflex test(s), if selected.
- Page 34 ▲ and ▼ keys to view the results for the other frequencies. If the Allegro was set to test for a reflex at all levels of the stimulus (see Reflex Autostop) press ► to view an additional display following the reflex graphs. This shows a summary of the levels and frequencies at which a reflex was detected.
- Page 35 ►. The message “Saving as last test” will be displayed briefly and the results will be saved in the “last test” memory. The results will remain available until a new test is started, even if the Allegro is turned off.
-
Page 36: Error Messages
• MAIN MENU (Return to the main menu) The results of the last test performed remain available even if the Allegro has been turned off. To view these results select VIEW THE LAST TEST from the main menu. After selecting the required ear, the tympanogram will be displayed. -
Page 37: Saving Results In The Database
GSI Allegro Tympanometer User Manual AVING ESULTS IN THE ATABASE To save the results of a test select SAVE RESULTS from the PROCESS RESULTS menu that is displayed on completion of a test. This option can also be accessed by selecting VIEW THE LAST TEST from the main menu and scrolling through the results using the ►... -
Page 38: Database Full
GSI Allegro Tympanometer User Manual To cancel saving the last test: • Delete any characters that have been entered. • Press and hold the ◄ key. ATABASE FULL A warning will be displayed if the database is full when attempting to save a test:... -
Page 39: Sending The Results To A Printer
Before attempting to print ensure the printer is fully charged, switched on, loaded with paper and ready to print. If the Allegro is in the cradle the data will be sent via the connecting cable. This operation is carried out automatically, although reference should be made to the appropriate guidance notes below. -
Page 40: Data Management
GSI Allegro Tympanometer User Manual ANAGEMENT Up to 32 patient records can be stored in the database of the GSI Allegro. Records can be listed, viewed, deleted, or printed using the DATA MANAGEMENT option of the main menu. DATA MANAGEMENT... -
Page 41: Delete Records
GSI Allegro Tympanometer User Manual Each entry shows: • Three-letter patient identifier entered when the test was stored; • Date and time of the test • Whether the test has been printed ( ) • Whether the test has been sent to a computer ( ) •... -
Page 42: Performing Daily Checks
GSI Allegro Tympanometer User Manual ERFORMING AILY HECKS The operation of the Allegro should be checked daily using the 4 in 1 test cavity assembly supplied with the instrument. Select the DAILY CHECK option in the main menu: DAILY CHECK... -
Page 43: Routine Maintenance
The device is not heat-resistant. Do not autoclave The Allegro is a precision instrument. Handle it carefully to ensure its continued accuracy and service. Use a soft damp cloth and mild detergent to clean the instrument panel and case when required. -
Page 44: Calibration And Repair Of The Instrument
ALIBRATION AND EPAIR OF THE NSTRUMENT GSI recommends that the Allegro is calibrated annually. Please contact your GSI distributor for details. If the instrument is to be used at elevations above 1000 m (3281 feet) above sea level re- calibration must be undertaken at the intended operating elevation. - Page 45 3048.0 2.9 ±0.1 WARNING The instrument should be returned to the GSI distributor for service and repair. There are no user-serviceable parts within it. When packing the instrument for shipping, please use the original shipping carton and packing materials. Place the instrument in a plastic bag before packing to prevent dirt and dust getting into the probe.
-
Page 46: Error Messages & Fault Conditions
Recharge the batteries before performing tests Powering down Other than after the specified power off delay, the Allegro may turn off because the internal batteries are spent. To replace the batteries, contact your GSI service center. AIRFLOW ERROR. Pump fault. If the fault persists, contact your GSI Cannot determine pump direction. - Page 47 This message should never be seen. Check all the Default configuration settings CONFIGURATION settings before taking any reloaded. Check before making new measurements. If the error persists, contact your GSI tests service center. WITHDRAW PROBE The probe has been moved during measurement. Re- insert the probe to repeat the test.
-
Page 48: Ordering Consumables And Accessories
ONSUMABLES AND CCESSORIES To order consumables, additional accessories and to replace detachable parts that have been damaged, please contact GSI or your GSI distributor for current prices and delivery charges. Some of the items available are listed below: Part Number... -
Page 49: Appendix - Menu Summary
GSI Allegro Tympanometer User Manual PPENDIX UMMARY Default values are shown in bold where appropriate. AIN MENU Menu Sub-menu MAIN MENU NEW TEST CONFIGURATION VIEW THE LAST TEST DAILY CHECK DATA MANAGEMENT SYSTEM INFORMATION ENU SELECTIONS Sub-menu Option Choices / Description... - Page 50 GSI Allegro Tympanometer User Manual REFLEX FREQUENCIES Selectable from 500, 1000, 2000 and 4000 REFLEX SELECTION ALWAYS MEASURE NEVER MEASURE ONLY IF PEAK FOUND PROMPT TO MEASURE REFLEX THRESHOLD Default is 0.03 ml REFLEX AUTO-STOP Default is YES. REFLEX POLARITY Choose whether a reflex trace is shown ascending (UP) or descending (DOWN).
- Page 51 GSI Allegro Tympanometer User Manual RELOAD DEFAULTS The options in this group are reset to their default values. Select ENGLISH, GERMAN, FRENCH, SELECT LANGUAGE SPANISH, PORTUGUESE or ITALIAN for operating language. CONFIGURATION All configuration options are reset to their (RELOAD DEFAULTS)
- Page 52 GSI Allegro Tympanometer User Manual SYSTEM INFORMATION Displays: Battery voltage Date calibrated Date of next calibration Instrument serial number Software version Current date and time D-0120695 Rev D 2022-06 Page 39...
-
Page 53: Appendix - Technical Specification
GSI Allegro Tympanometer User Manual PPENDIX ECHNICAL PECIFICATION Tympanometry Instrument type Meatus compensated tympanometer Analysis performed Admittance peak level (in ml); Pressure of same; Gradient (in daPa); Ear Canal Volume (ECV) @ 200 daPa Probe tone levels and accuracy 226Hz +/- 2%; 85dB SPL +/-2dB over range 0.2ml... - Page 54 GSI Allegro Tympanometer User Manual Number of reflex levels (see Acoustic Four: 100dB with 5dB or 10 dB steps; 95dB, 90dB Reflex Measurement) or 85dB with 5 dB steps Reflex analysis Reflex pass/fail at each level tested; maximum amplitude of each reflex (seen on printed report &...
- Page 55 GSI Allegro Tympanometer User Manual Languages Operating Languages English, German, French, Spanish, Portuguese or Italian Printing Supported printer Sanibel MPT-II Interface Wired connection to cradle Information printed Space for patient & clinician’s details, Tympanogram analysis parameters, Tympanogram, Reflex analysis parameters, Reflex...
- Page 56 GSI Allegro Tympanometer User Manual Physical Display 128 x 64 pixels / 8 lines of 21 characters Dimensions 230mm (L) x 115mm (W) x 70mm (H) Probe: 30 mm x 22 mm x 22 mm Total Weight (handset and cradle)
-
Page 57: Equipment Classification
GSI Allegro Tympanometer User Manual QUIPMENT CLASSIFICATION The GSI Allegro Tympanometer is classified as a Class IIa device under Annex IX (Section 1) of the Medical Device Regulation (EU) 2017/745. Type of protection against electric shock Internally Powered Degree of protection against electric shock... - Page 58 GSI Allegro according to the EMC information presented in this appendix. The GSI Allegro has been tested for EMC emissions and immunity as a standalone instrument. Do not use the device adjacent to or stacked with other electronic equipment. If adjacent or stacked use is necessary, the user should verify normal operation in the configuration.
- Page 59 GSI Allegro Tympanometer User Manual Guidance and Manufacturer’s Declaration - Electromagnetic Emissions The GSI Allegro is intended for use in the electromagnetic environment specified below. The customer or the user of the GSI Allegro should assure that it is used in such an environment.
- Page 60 GSI Allegro Tympanometer User Manual Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The GSI Allegro is intended for use in the electromagnetic environment specified below. The customer or the user of the Allegro should assure that it is used in such an environment.
- Page 61 To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the GSI Allegro is used exceeds the applicable RF compliance level above, the GSI Allegro should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the GSI Allegro.
- Page 62 Recommended Separation Distances between Portable and Mobile RF Communications Equipment and the GSI Allegro The GSI Allegro is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the GSI Allegro can help prevent electromagnetic interferences by...
- Page 63 GSI Allegro Tympanometer User Manual Test Specifications for Enclosure Port Immunity to RF wireless communications equipment Test Band a Immunity Maximum power Distance Service a Modulation b frequency Test Level (MHz) (MHz) (V/m) Pulse modulation b 380 –390 TETRA 400...
- Page 64 The operator must not touch the connected equipment and the patient at the same time as this would result in an unacceptable hazard. Refer to GSI at the address given on the front of this user manual if advice is required regarding the use of peripheral equipment.







Need help?
Do you have a question about the ALLEGRO and is the answer not in the manual?
Questions and answers