Summary of Contents for Yuwell YX310
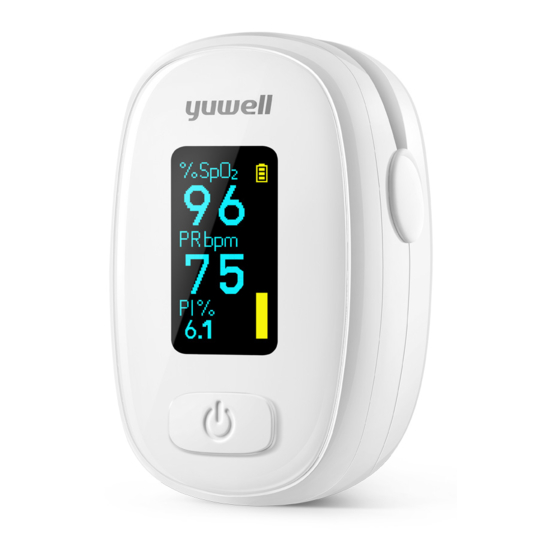
- Page 1 YUWELL® Finger Pulse Oximeter YX310 Please read the user manual carefully before using!(The picture is for reference only, please refer to the actual product.)
- Page 2 Warning 1.Warning,Do not modify this equipment without authorization of manufacturer. 2.Warning,Keep away from the wet medical equipment such as drip or other similar liquid simulation as far as possible. 3.Warning, Check the sensor site every half an hour to ensure adequate blood circulation, intact skin, and appropriate sensor location.
- Page 3 oxide to avoid the risk of explosion. 15.Caution,When using this product, you only need to fix it on your fingers. Excessive pressure on your fingers may cause skin damage. 16.The maximum skin surface temperature is below 41℃(106℉) when measured in a 35℃(95℉)environment, which has been verified by measuring the skin surface temperature via a Finger Pulse Oximeter under the reasonable worst conditions.
-
Page 4: General Description
26.Caution, The display may not read clearly when exposed to direct sunlight or bright light. 27.Caution, Do not use a functional tester to evaluate the accuracy of the Finger Pulse Oximeter. The functional tester shall only be used to check whether a unit is working properly. -
Page 5: Measurement Principle
The YUWELL® Finger Pulse Oximeter features in small volume, low power consumption, convenient operation and portable. It is only necessary for patient to put one of his fingers into a fingertip photoelectric sensor for measurement, and then the screen will display the measured value of pulse oxygen saturation and pulse rate. -
Page 6: Indication For Use
Bluetooth Indication for Use Intended use: The YUWELL® Finger Pulse Oximeter is a non-invasive, non-sterile, reusable, spot checking device which can measure and display SpO pulse rate through finger. It is intended for adults and children (weight > 30kg) and is expected for home and hospital inspection. -
Page 7: Technical Parameters
± 1% when measured under indoor nature light / existing lighting and measured in the dark room. 6.The product will automatically shut down when there is no signal detected for about eight seconds. 7.Dimension(YX310):60mm*38mm*35mm(LWH),Weight:38g approximately (without batteries). 8.Working Environments: Ambient temperature: 5℃~40℃;Relative humidity: ≤80%; Atmospheric pressure: 860hPa~1060hPa. -
Page 8: Technical Description
15.TYPE BF APPLIED PARTS 16.Degrees of protection provided by enclosures (IP code):IP22. 17. Description of oximeter application management YX310 is equipped with Bluetooth function. Bluetooth communication protocol module enables the oximeter to be equipped with Bluetooth connection and the function of date exchange ,which does not involve patient privacy, mainly including pulse rate, blood oxygen and other information. - Page 9 : accuracy. n: test sample quantity. : measured value of pulse oxygen saturation during the first measurement using the finger pulse Oximeter. : reference value of pulse oxygen saturation during the ith measurement using the carbon-monoxide-blood-gas analyzer. 4. Characteristics of Population under Clinical Research The group of healthy adult volunteers for the study consisted of 12 subjects - 4 women and 8 men, The ages ranged from 18 to 50 years.
-
Page 10: Product Properties
Product Operation Scope The YUWELL® Finger Pulse Oximeter is designed for fingers(not thumb) between 0.3 and 0.9 inch (0.8-2.3cm) thick. And the finger shall be inserted into the sensor position which is in the middle of the device. -
Page 11: Lanyard Installation
damage might occur to device. Please put or remove batteries in right order, or it will damage the device bracket. Please remove the battery if the oximeter is not used for long time. 3. Install as the figures show. (See Figure) Remove the battery from the product if it is not required for extended periods of time in order to avoid damage to the oximeter resulting from a leaking battery. -
Page 12: Cleaning And Disinfecting
Cleaning and disinfecting This product is a reusable non-sterile device. Please clean and disinfect according to the following methods. Warning: 1.Never immerse or soak the oximeter. 2.We recommend cleaning and disinfecting the oximeter before or after each use, or in accordance with the policies established by the hospital, to avoid long-term damage to the oximeter and avoid cross-infection. - Page 13 Possible cases and solutions Warning: Caution: Oximeter cover can only be opened by a professional maintenance staff. No internal parts require opening by end users. If you are not sure about the measurement precision, please use other methods to check patient's pulse, to determine whether oximeter works.
-
Page 14: Electromagnetic Interference
Electromagnetic interference The EM environment for this product is the home healthcare environment and professional healthcare facility environment. The essential performance of this product is the accuracy of SpO and pulse rate (SpO Accuracy: ±2% in the range of 70%-100% of SpO , No definition for SpO under 70%;Pulse rate: 25bpm~250bpm, accuracy: ±1% or ±1bpm(larger)).When used directly near strong electromagnetic interference (for example: near mobile... - Page 15 Immunity Measurements IEC/EN 61000-4-2 Electrostatic Discharge Pass IEC/EN 61000-4-3 Radio-frequency Electromagnetic Field Pass IEC/EN 61000-4-4 Fast transients, Common Mode IEC/EN 61000-4-5 Surges IEC/EN 61000-4-6 Radio-frequency Common Mode IEC/EN 61000-4-8 Power-frequency Magnetic Fields Pass IEC/EN 61000-4-11 Voltage Dips and Interruptions Table 1 - For all ME EQUIPMENT and ME SYSTEMS Guidance and manufacture’s declaration –...
- Page 16 Table 5 - For all ME EQUIPMENT and ME SYSTEMS Guidance and manufacturer's declaration – Power-frequency Magnetic Fields IMMUNITY test IEC60601 test level Compliance level Result Power frequency (50/60Hz) 30 A/m 30 A/m Pass magnetic field 50Hz or 60Hz 50Hz and 60Hz IEC 61000-4-8 Table 6 - Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment...
- Page 17 LTE Band 7 5240 Pulse 5100-58 WLAN 802.11 5500 modulation 217Hz 5785 NOTE:If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the transmitting antenna and the ME EQUIPMENT or ME SYSTEM may be reduced to 1 m. The 1 m test distance is permitted by IEC 61000-4-3.
-
Page 18: Warranty Card
Manufacturer Address:No.1 Baisheng Road Development Zone, Danyang, Jiangsu 212300 CHINA For information about any YUWELL product, please contact:www.yuwell.com Due to the limited size of the label, the font is too small, please put it at a suitable location for viewing. All specifications and product configurations are subject to change without notification.

















Need help?
Do you have a question about the YX310 and is the answer not in the manual?
Questions and answers