Subscribe to Our Youtube Channel
Summary of Contents for OsteoSys SONOST-2000
- Page 1 User’s Manual Model: SONOST-2000 REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Doc Version : 12.2_MDR (2022.09.05) http://www.osteosys.com OsteoSys Co., Ltd.
- Page 2 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 User’s Manual CAUTION ! You must be well acquainted with this manual before using it. This manual should be placed where the user could read it whenever necessary.
- Page 3 Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Thank you for purchasing SONOST-2000 Ultrasound Bone Densitometer. To ensure safe operation and long-term performance stability, it is essential that you fully understand the functions and operating, maintenance instructions by reading this manual before operating the equipment.
- Page 4 OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 The symbols which are shown in this manual or SONOST-2000 The noticed information which should be concerned with explanation in this manual The noticed information when the device is operated...
- Page 5 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 The protecting device from external electromagnetic wave This device can be affect from external electromagnetic wave which is related to precision and operation. When you using this device, we strongly recommend you, avoid from other device from protect electromagnetic wave.
- Page 6 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 - Auxiliary devices should be maintained clean, in working conditions. - Devices should be positioned in their proper positions, so as not to interfere with worker or patient movement.
- Page 7 Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 The model SONOST-2000 is intended for use in the electromagnetic environment specified below. The customer or the user of the model SONOST-2000 should assure that it is used in such an environment. A or Contents...
- Page 8 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 • Organ transplantation • Prolonged immobilization • Conditions associated with secondary osteoporosis, such as gastrointestinal malabsorption or malnutrition, sprue, osteomalacia, vitamin D deficiency, endometriosis, acromegaly, chronic alcoholism or established cirrhosis, and multiple myeloma •...
- Page 9 Ages: 20 – 100 years old Patient b) The patient condition: Osteopenia, Osteoporosis or Normal healthy bone SONOST-2000 has silicon balloon on the probe part which can keep the water inside of balloon as media for path of ultrasound wave more Disposable device efficiently.
- Page 10 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Voltage fluctuations/ Complies flicker emissions IEC 61000-3-3...
- Page 11 Guidance and manufacturer’s declaration – electromagnetic immunity The model SONOST-2000 is intended for use in the electromagnetic environment specified below. The customer or the user of the model SONOST-2000 should assure that it is used in such an environment. Electromagnetic environment – guidance...
-
Page 12: Table Of Contents
3.1.7 Selection of the foot supporter....................30 Using S/W ............................32 3.2.1 Progress Table of Program ......................32 3.2.2 Using Program .........................33 Chapter 4. Maintenance and Repair of SONOST-2000 ................70 Resolution to Problems ........................70 Maintenance and Repair ........................71 4.2.1 Cleaning ..........................71 4.2.2 Stockpile and Replacement of Articles ..................72 4.2.3 Storage .............................73... - Page 13 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Labels ..............................82 Chapter 6. Product Warranty ........................82 Chapter 7. Security .............................85...
-
Page 14: Chapter 1. Introduction
1 Chapter 1. Introduction 1.1 General Product Information Osteoporosis is one of the serious diseases. This device is a bone densitometer which estimates a bone mineral density of the calcaneus by ultrasound. It takes about 15 seconds to measure the density and to display the shape of ultrasonic wave by computing simulation on the monitor. -
Page 15: Chapter 2. Configuration Of Sonost-2000
When you give the appropriate commands in SONOST-2000 software, ultrasonic waves are generated. The generated ultrasonic waves pass through the Patient’s calcaneus and the electric signal is treated by SONOST-2000 algorithm. SONOST-2000 measures speed of sound(SOS) and the frequency-dependent... -
Page 16: Composition Of Sonost-2000
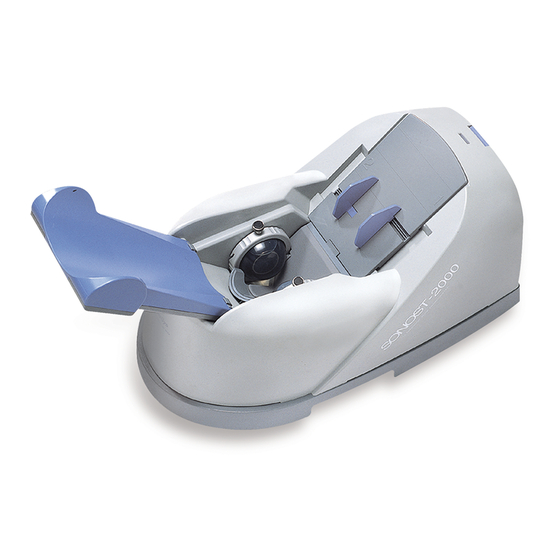
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 2.1 Composition of SONOST-2000 2.1.1 Shape and Components of SONOST-2000 Name Function TOP COVER Protection for internal circuit BOTTOM PLATE Fixation for device position & top cover... -
Page 17: Accessories List
OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Activate SONOST-2000 by pressing a key on the keyboard according to the instructions shown on the monitor. The manual shows keys required to activate SONOST-2000 as shown below. 2.1.3 Accessories List... - Page 18 OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Check the following accessories before installing the system. If they are not in good condition, contact to OsteoSys or its authorized dealer for this service. Names of Products Number...
-
Page 19: Articles List
DOC. No. : OT01-2R7123 2.1.4 Articles List Check the following accessories before installing the system. If they are not in good condition, contact to OsteoSys or its authorized dealer for this service. 4.2.2 Stockpiles and Replacement of Articles Names of Products... -
Page 20: Installation Of Sonost-2000
DOC. No. : OT01-2R7123 2.2 Installation of SONOST-2000 NOTE ! Since SONOST-2000 is made up of precise components, you should install the product according to instructions below. Do not install and keep SONOST-2000 at Do not install or keep SONOST-2000 in excessively high or low temperatures. -
Page 21: Before Turning On Power
(1) Connection of Power & USB Cable(with the device) Plug in the cable. Plug into the connector. When you detach the cable from the main body of SONOST-2000, do not hold the cable and instead hold the connector. Otherwise the wire could be down. - Page 22 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123...
-
Page 23: Chapter 3. Using Sonost-2000
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3 Chapter 3. Using SONOST-2000 Using H/W 3.1.1 Applying the Balloon to the Measuring Device Clean the surface of the probe with an alcohol pad. Attach the balloon to the probe holder. - Page 24 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Take water into the balloon using an injector until it is full(65~70cc). Close it with the stopper. Tap the balloon to remove bubbles inside of the balloon.
-
Page 25: Using The Qc Phantom
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.1.2 Using the QC phantom 3.2.2.8 Dailytest of SONOST-2000 Insert the standard block positioner. Apply gel on the both side of the QC Phantom. Put the QC Phantom between the balloons and press the phantom until the phantom touches the bottom. -
Page 26: Patient Measurement Procedure
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.1.3 Patient Measurement Procedure Put the foot positioner in accordance with patient’s foot size - Ex) Using the #1foot positioner - Ex) Using the #2 foot positioner... - Page 27 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Apply plenty of gel on the both side of patient’s heel. Insert patient’s foot Let a patient move his foot up and down for the balloons to be covered with ultrasound gel sufficiently.
-
Page 28: Disposing Water
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.1.4 Disposing water Dispose water inside the measurement device ▶ Replacement period : 1 ~ 3(week) ▶ If there is air in the balloons, fill up water promptly. -
Page 29: Correct Position For Patient's Foot & Body
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 No Good CAUTION ! To prevent damage to skin and cells, the uses has to use commercially available ultrasound gels that have passed ISO 10993-5(Cytoxicity), ISO 10993-10(Skin sensitization) and ISO 10993-23(Skin irritation) tests. -
Page 30: Selection Of The Foot Supporter
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Patient Device 3.1.7 Selection of the foot supporter Put the foot supporter according to the below guide. The section is arranged for each size of the foot. - Page 31 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (foot supporter 1) (foot supporter 2) (foot supporter P)
-
Page 32: Using S/W
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Using S/W 3.2.1 Progress Table of Program... -
Page 33: Using Program
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2 Using Program 3.2.2.1 Setup program (1) Execute the Setup.exe file for installation. (2) Click [Next] (3) Click [Next] (4) Click [Next]... - Page 34 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (5) Click [Next] (6) Click [Next]...
- Page 35 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (7) Click [Finish], Program installation is finished.
- Page 36 3.2.2.2 Setup USB Driver ※ The following is the instruction for Windows XP driver (1) Connect the USB cable from PC to SONOST-2000, Hardware Wizard will start. (2) Select [Install from a list or specific location(Advanced)] and Click [Next] (3) Select [Include this location in the search] and Click [Browse].
- Page 37 DOC. No. : OT01-2R7123 (4) Select the folder that contains the driver files and Click [Next] ' My computer > C > Program Files > OsteoSys > SONOST-2000 > USB Driver > WinXP ' (5) Select [SONOST-2000+XP] and Click [Next]...
- Page 38 DOC. No. : OT01-2R7123 (6) Click [Continue Anyway] (7) Click [Browse] (8) Go to the folder indicated below, select a SonostXP.sys file and click [OK]. ' My computer > C > Program Files > OsteoSys > SONOST-2000 > USB Driver > WinXP '...
- Page 39 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (9) Click [Finish] (10) You must check if [SONOST-2000+XP] is appeared under Universal serial bus controller. (Exclamation mark[!] must be removed)
- Page 40 ※ If not installed properly, you can refer to the instructions below. ※ The following is the instruction for Windows 7 driver How to install the SONOST-2000 driver for Windows 10 in next chapter. ※ The following is the instruction for Windows 10 driver (1) Insert the SONOST-2000 driver CD.
- Page 41 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (6) Connect the USB cable from PC to SONOST2000. (7) The drivers are installed automatically and the following window appears, click Close. (8) You must check whether [s2k_usb Device] is shown under Universal Serial Bus devices.
- Page 42 DOC. No. : OT01-2R7123 3.2.2.3 Execution of Program and Configuration of the Incipient Environment CAUTION ! If you hear any strange noise from the screen, switch it off and contact to OsteoSys or its Authorized dealer. (1) Turn on the power switch.
- Page 43 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (4) Hospital : Set the name, address and phone number of your hospital. 3.2.2.4 Registration & Deletion of Users (1) As soon as you configure the user environment, the “Doctor Selection” window appears as shown below.
- Page 44 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (2) If you are a new user(doctor), click the “New Doctor” button in the “Doctor Selection” window, and then the “New Doctor” window appears. If you enter your information(name, phone number, department and password) and click the “OK”...
- Page 45 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (3) If you want to delete registered users, select a desired user and click the “Doctor Delete” button of the “Doctor Selection” window. If there are two or more registered users, the message box “Patients Shifting”...
- Page 46 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (4) If you click the “OK”46or “NO” button, the message box “Surely delete doctor?” inquiring whether to delete the user surely appears. If you click the “OK” button the user information is deleted.
- Page 47 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.5 BQI Measurement < Registration & Retrieval of Patients > (1) If you click the “BQI Measurement” button in the default screen, you can move to the “Patient List”...
- Page 48 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (2) If you want to register new patients, click the “New Patient” button on the bottom of the “Patient List” screen. Then the “New Patient” window you can enter the information of new patients appears.
- Page 49 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 < Measurement of Patients > (1) If you want to measure BQI, select a desired patient using the mouse in the “Patients List” screen and click the “Measurement” button on the right side of the screen. Then the “Measurement”...
- Page 50 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (3) When ultrasonic waves are generated, the word on the “Measurement Start” button is changed into “Measurement Stop”. In case of emergency, click this button, and you can stop measuring.
- Page 51 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (5) If you need to write or modify comment, click the “Comment Edit” button on the bottom of the screen. Then the “Comment Edit” window, you can write or modify comment.
- Page 52 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.6 Revision & Deletion of Patient’s Information (1) If you want to revise the registered patients’ information, select a desired patient using the mouse and click the “Info Change” button in the “Patients List” screen.
- Page 53 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (4) After selecting a desired patient, click the “Patient Delete” button on the bottom of the screen. Then the message box “Patient Deletion” inquiring about whether to delete the information surely appears and if you click the “OK”...
- Page 54 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.7 Inquiry & Deletion of Clinical History of Existing Patients (1) If you want to retrieve the clinical history of existing patients, select a desired patient and click the “Patient Info”...
- Page 55 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 ① If you click the “Print Preview” on the right side, the result you selected with a mouse appears in the “Patient’s Info” screen. ② If you click the “Print Preview” on the left side, all results of the selected patient...
- Page 56 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 [ Print Sample ① ]...
- Page 57 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 [ Print Sample ② ]...
- Page 58 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (2) After revising patients’ information(menopause state, taking drug, etc.), if you click the “Result Update” button in the result screen, the message box “Result Updating” inquiring about whether to update the information surely appears. If you click the “OK”...
- Page 59 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.8 Dailytest of SONOST-2000 (1) Make sure to dailytest the system to reduce a margin of error and to get accurate values in times of examining bone density. It is to raise credibility of examination results through checking of system reliability on a daily basis.
- Page 60 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (3) When ultrasonic waves are generated, the word on the “Daily Test Start” button is changed into “Daily Test Stop”. In case of emergency, click this button and you can stop daily testing.
- Page 61 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.9 Data Backup (1) If you need to save the data, click the “Backup” button in the default screen. If you click the button, the default screen of “Backup” menu appears as shown below.
- Page 62 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 in the PC. (4) Clear : If you click this button, the message box “Surely clear doctor?” inquiring whether to delete the user surely appears. If you click the “OK”...
- Page 63 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 All records related the DB are written in this field. After selecting the record and clicking the “OK” button in this screen, the DB is recovered.
- Page 64 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.10 Configuration of Environment (1) If you want to configure the user environment again, click the “Configuration” button in the default screen. There are four items from “Settings” to “Doctor” in this menu.
- Page 65 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (4) Doctor : You can set your name, phone number and department. If you click the “PW Change” button, you can change your password. 3.2.2.11 Inactivation of SONOST-2000 If you click the “Exit”...
- Page 66 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 3.2.2.12 FRAX FRAX Mode predicts the risk of fracture within the next 10 years using the patient's lifestyle and medication use. The FRAX results predict the risk of femoral fractures and major osteoporotic fractures (vertebral, wrist, femur, and shoulder fractures) within 10 years.
- Page 67 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (2) Entering from Patient Info In the Patient Info screen, select FRAX button and input FRAX information. Frax Screen configuration is as follows. Frax Screen...
- Page 68 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 ① It is possible to select individual risk factors of the patient to predict fracture risk. More than 3 drinks a day If you drink more than three drinks a day, please check the box.
- Page 69 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 (1) Frax Report #The report contents are for reference only.
-
Page 70: Chapter 4. Maintenance And Repair Of Sonost-2000
Is the hardened gel ticked on the surface of the abnormal balloons 4.2.1 Cleaning Is some material on the surface of QC The balloons tears easily phantom? CAUTION ! If you cannot resolve the problem, contact to OsteoSys or its authorized dealer. -
Page 71: Maintenance And Repair
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 4.2 Maintenance and Repair 4.2.1 Cleaning CAUTION ! Turn off the power before cleaning the system. Product Example Instructions After using the device, remove the balloons from the device and wash with water. -
Page 72: Stockpile And Replacement Of Articles
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 4.2.2 Stockpile and Replacement of Articles Make sure to keep following articles of consumption all the time. 2.1.5 Articles List Articles Specifications Purchase ones at our authorized dealer when it needs to be used. -
Page 73: Storage
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 4.2.3 Storage 1) Unplug the power cord. 2) Dust on the product can damage the system. After use, make sure to cover it with a vinyl cover. -
Page 74: Safe Use Of Sonost-2000
OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 4.3 Safe Use of SONOST-2000 4.3.1 Safety Rules The followings are instructions for safe use of the product Make sure to comply with the instructions when you activate the system. -
Page 75: Cautions Related To Use Electronic Medical Equipment
4.3.1 Safety Rules 1 Nobody can handle SONOST-2000 except for doctors and authorized personnel 2 Comply with followings in times of installation of SONOST-2000. Do not install it in following places. A) Places where the product is exposed to water vapor B) Places where the product is exposed to spray or splashing water. - Page 76 OsteoSys or its authorized dealer for assistance. G) Before electrically or mechanically connection any other manufactures’ devices to SONOST-2000, contact OsteoSys or its authorized dealer for instructions H) Do not operate the product improperly when you use it with other products.
- Page 77 Otherwise, abnormal images may result. An independent circuit and a safely grounded outlets are strongly recommended for SONOST-2000. Poor or abnormal images may occur if the system shares a power source with other electrical or electronic equipment.
- Page 78 E) Make sure to clean the product so that users can use it immediately 6 If the product is out of order, do not try to repair it for yourself. 7 Do not modify use the product. If any modification is desired, ask OsteoSys or its authorized dealer for this service.
-
Page 79: Chapter 5. Specifications & Software Updates
User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 5 Chapter 5. Specifications & Software Updates 5.1 Specifications *General Classification Ⅰ Class , Type B Applied part *Device Dimensions (W x D x H) 289mm × 602mm × 361mm Weight 7.8 Kg... -
Page 80: Software Updates
Install system in a clean, well-ventilated area. Excessive dust and other airborne debris Dust, Fumes, Airborne debris contaminants can impair sensitive parts. We recommend instituting a “No Smoking” policy in system area. 5.2 Software Updates 1. Updates to SONOST-2000 software are available at our... - Page 81 OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 homepage (http://www.osteosys.com). 2. Updates to SONOST-2000 software are free, regardless of the product warranty. 3. Updates to SONOST-2000 software are irregular, we will inform you through your email whenever SONOST-2000 software needs to be updated.
- Page 82 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 5.3 Labels 6 Chapter 6. Product Warranty...
- Page 83 (6) Cosmetic defects or deterioration will not be refinished or replaced. The costs of replacement of fuses are not covered. (7) OsteoSys Co., Ltd. will not be responsible for any loss, damage, or injury resulting from delay in rendering service under this warranty.
- Page 84 OsteoSys Co., Ltd. will replace the defective product at no cost to you. OsteoSys Co., Ltd. will pay the shipping and insurance costs of product sent to you.
- Page 85 Security SEC.1. Introduction This section describes the security features, functionality and management requirements of OsteoSys software. The manual is intended to assist medical facilities in using the system in a manner that protects the privacy and security of patients and to perform their work in accordance with national regulatory requirements.
- Page 86 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 • How to secure access • When to grant access • access information Access control includes electronic as well as physical aspects, and includes authentication and authorization.
- Page 87 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 Audit Log will be added every data handling action. SEC.5. Certification Authentication is the process of providing an individual's identity and is a key component of an access control system.
- Page 88 Customers should visit the OsteoSys website for up-to-date information about vulnerability information and the impact on software. Customers need to install validated Microsoft security software patches. 1. OsteoSys software computing system holders must apply a validated Microsoft security software patch for their Windows operating system version.
- Page 89 When the user wants to update the system, please contact OsteoSys to receive appropriate service. SEC.11. Third-Party Components in Product Lifecycle Roadmap OsteoSys provides information security throughout the entire life cycle of the product from its launch to discontinuation. Information security requirements should also consider: a) the level of confidence required towards the claimed identity of users, in order to derive user authentication requirements;...
- Page 90 And OsteoSys provides Cyber security product upgrades. As soon as possible, third-party security patches need to be installed in medical products in accordance with regulations requiring. OsteoSys Provides product security patch upgrades in a unified working manner by installation/field service personnel. NOTE ! Third party component updates will proceed with the distribution of patch files from OsteoSys during product warranty.
- Page 91 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 CAUTION ! The user needs to close not needed network ports via a firewall to protect the whole system. SEC.13. Unauthorized network access Patient health care today relies heavily on IT to electronically collect, process, distribute, display and store patient data.
- Page 92 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 SEC.15. System security You can set up a screensaver with password protection to block access to the system after a period of inactivity. CAUTION ! Without appropriate logoff, leaving the work spot could be dangerous.
- Page 93 DOC. No. : OT01-2R7123 SEC.18. HEALTH DATA Storage and Confidentiality To provide security of health data stored in products or media, OsteoSys uses a database and a specific file system. In order to access, view, and modify patient health data, it is necessary to obtain access to the database and to interpret the specific file system.
- Page 94 CAUTION ! Do data backup at least every week. OsteoSys could not recover the data not performed latest data backup when the disaster occurred. SEC.23. Data Backup and Disaster Recovery The UI Program backs up data and database once a week.
- Page 95 SEC.25. Remote service Often, the most efficient and effective way to service OsteoSys is to access the system remotely. Every effort is made to ensure the security of these connections. OsteoSys software requires separate TeamViewer remote access. In no case is the instrument activated remotely.
- Page 96 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123 • Remote service connection via TeamViewer SEC.27. Network Interface Technical Specification Connection name PC Motherboard NIC Physical network connection type IEEE 802.3 10/100 / 1000BASE-T Ethernet...
- Page 97 WARNING ! When DICOM transmission is succeeded but transmitted data is malformed, first contact the network manager and the DICOM server provider. OsteoSys has verified DICOM features with other DICOM systems. SEC.30. Required Characteristics The network must meet the specific requirements for a subset of the functions, use cases required by users in the responsible organization, and all of the above flows related to the workflow.
- Page 98 User’s Manual (Confidential) OsteoSys Co., Ltd. Model Designation : SONOST-2000 DOC. No. : OT01-2R7123...





Need help?
Do you have a question about the SONOST-2000 and is the answer not in the manual?
Questions and answers