Summary of Contents for NuvoMed TTM-6/0740
- Page 1 TTM-6/0740 Low-Frequency Therapy Instrument Instruction Manual Please read all instructions carefully and retain for future use...
-
Page 2: Table Of Contents
Know your Device Indication for Use Medical Disclaimer Precautions Important Safety Information Battery Warning Preparation Package Content Parts About Therapy Machine Set up your Device Getting Start After Used Instruction of Use Care and Maintenance Troubleshooting EMC Declaration 10-12 Specification Warranty Information... -
Page 3: Know Your Device
Know your Device Indications for Use The Low-Frequency Therapy Instrument is a product that adopts modern electronic science and technology to produce low-frequency electrical pulse and transmit that through the skin to the underlying peripheral nerves by electrodes, in order to reach the therapeutic aim. This device used for temporary relief of pain associated with sore and aching muscles in the shoulder, waist, back, arm, leg and foot, due to strain from exercise or normal household and work activities. -
Page 4: Important Safety Information
Important Safety Information 1. Never use this product along with other medical electronic instruments, such as cardiac pacemaker, artificial heart-lung which are used to maintain life, and electrocardiograph. Otherwise it may cause danger. 2. If the user is using high frequency surgical equipment and this therapy instrument at the same time, then there might be burn in the place where the electrode patch is pasted to or damage to the instrument;... -
Page 5: Battery Warning
13. transthoracically and transcerebrally; 14. over swollen, infected, or inflamed areas or skin eruptions; 15. over, or in proximity to, cancerous lesions. 16. Do not arbitrarily disassemble, repair and reform this instrument, otherwise it might cause malfunction or electric shock accidents. 17. -
Page 6: Preparation
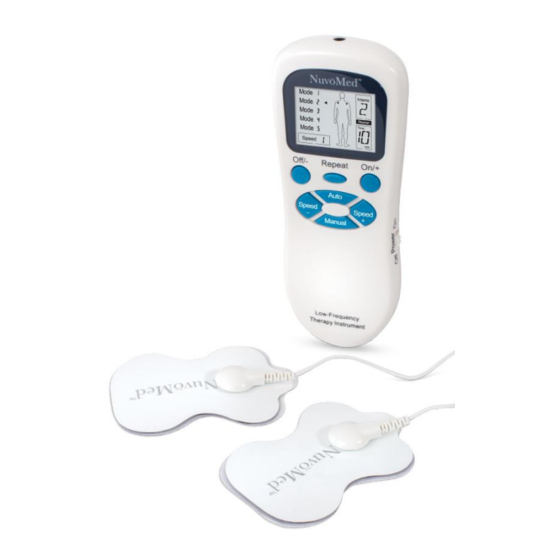
Package Content Low-Frequency Therapy Instrument Machine Pair of Electrode Pads Connecting Cable Storage Pouch 2 AAA Batteries (Included) Parts About Therapy Machine Therapy Machine User can select appropriate treatment mode and other parameters, such as intensity, through function buttons (shown below) on the host and know about the current status through LCD at any time. -
Page 7: Set Up Your Device
Set Up Your Device 1. Open battery cover and insert 2 AAA batteries into the battery compartment according to polarity. Caution: When the intensity stimulation is very weak, it means the battery is low and a new battery should be replaced. 2. -
Page 8: Instruction Of Use
Set Up Your Device Instruction for Use 1. Press “On/+” button to power on and start working procedure, the instrument enters into automatic combination mode 5 (intensity is 1st level, timing for 15 minutes), LCD displays corresponding contents. 2. Press “On/+” button after the instrument has been powered on to enhance intensity which is divided into 9 levels. -
Page 9: Care And Maintenance
For regular maintenance, this device only needs to be wiped gently with a soft, dry cloth. Never immerse this device or any components in water. • Do not carry out repairs of any kind yourself. If a defect occurs, please contact your local authorized distributor. -
Page 10: Emc Declaration
EMC Statement This device needs special precautions regarding EMC and need to be installed and put into service according to the EMC information provided in the accompanying document. Portable and mobile RF communications equipment can affect this device. Warning: • The use of accessories, transducers and cables other than those specified with the exception of transducers and cables sold by the manufacturer of this device as replacement parts for internal components, may result in increased emissions or decreased immunity of this model. - Page 11 Immunity IEC 60601 Compliance Electromagnetic Test Test Level Level Environment - Guidance Electrostatic + 8 kV + 8 kV Floors should wood, contact contact discharge (ESD) concrete, or ceramic tile. + 2 kV, + 2 kV, floors covered with IEC 61000-4-2 + 4 kV, + 4 kV, synthetic material, the relative...
- Page 12 Immunity Test IEC 60601 Compliance Level Electromagnetic Test Level Environment - Guidance 3 V/m Portable and mobile RF Not applicable communications equipment should 150kHz to 80MHz be used no closer to any part of the 6 Vrms in ISM models including cables, than the bands Conducted RF 10 V/m...
-
Page 13: Specification
Model TTM-6/0740 Dimensions – Therapy Machine 147mm (L) x 59mm (W) x 27.5mm (D) Dimensions – Electrode Pads 83mm x 59mm x 6mm Dimensions – Connecting Cable Cable Length: 1.2m Weight – Therapy Machine 2.7oz Power 2 AAA (Included) Number of Channels / Modes... -
Page 14: Warranty Information
No charge will be applicable for such repair or replacement. SERVICE AND REPAIR: If service is required for this product, you should first contact Nuvomed Inc. Customer Service at info@nuvomed.us or by calling Toll-Free Number 1 (866) 815-4714, Monday to Friday 10am to 6pm EST.





Need help?
Do you have a question about the TTM-6/0740 and is the answer not in the manual?
Questions and answers