Table of Contents
Advertisement
CAPTUS
4000e
®
Manual Stock No. 9250-0151
August 12, 2020
Rev. K
2020/12/08
THYROID UPTAKE SYSTEM
OWNER'S MANUAL
Mirion Technologies (Capintec), Inc.
7 Vreeland Road
Florham Park, NJ 07932 USA
Phone (800) ASK-4CRC
Fax (201) 825-1336
The authorized representative located within the Community is:
Atlantico Systems Ltd.
34 Oldfield, Kingston, Galway, Republic of Ireland
©
Copyright
2020 Capintec, Inc.
®
CAPTUS
is a registered trademark of Capintec, Inc.
ALL RIGHTS RESERVED
Advertisement
Table of Contents

Summary of Contents for Capintec CAPTUS 4000e
- Page 1 Phone (800) ASK-4CRC Fax (201) 825-1336 The authorized representative located within the Community is: Atlantico Systems Ltd. 34 Oldfield, Kingston, Galway, Republic of Ireland © Copyright 2020 Capintec, Inc. ® CAPTUS is a registered trademark of Capintec, Inc. ALL RIGHTS RESERVED...
- Page 2 CAPINTEC, INC. CAPTUS ® 4000e This page intentionally left blank.
-
Page 3: Table Of Contents
TRADEMARKS ....................3 GENERAL Thank you for purchasing the Capintec line of Thyroid Uptake Measurement Systems. Every effort has been made to ensure that the information in this document is complete, accurate, and up-to-date. Mention of products manufactured by other companies does not necessarily constitute endorsement by Capintec, Inc. -
Page 4: Medical Equipment Safety Classification
Increase the separation between the equipment and the affected device. • Plug the unit into an outlet on a circuit different from that which the affected device is connected. If this fails to correct the problem, please contact Capintec’s only Authorized Service Center. PREFACE August 20... -
Page 5: Installation
Understand all warning and caution labels as explained in the CHAPTER 1: SAFETY before operating this equipment. TRADEMARKS CAPTUS ® 4000e and CII are registered trademarks of Capintec, Inc. Microsoft ® and MS Windows ® are U.S. registered trademarks of the Microsoft Corporation. - Page 6 CAPINTEC, INC. CAPTUS ® 4000e TABLE OF CONTENTS PREFACE SAFETY ..........................1-1 GENERAL........................1-1 SYMBOL DEFINITIONS ....................1-1 WARNING AND CAUTION LABELS ................1-2 CAUTIONS AND NOTES ....................1-3 ELECTROMAGNETIC COMPATABILTIY TEST DATA........... 1-7 GENERAL SAFETY TIPS ..................... 1-11 FUNCTIONAL & TECHNICAL DESCRIPTION ..............2-1 INTENDED USE ......................
- Page 7 CAPINTEC, INC. CAPTUS ® 4000e Life Time (End of Life) ....................2-10 GENERAL OPERATING INSTRUCTIONS ................. 3-1 INTRODUCTION ......................3-1 WINDOWS OPERATING CONVENTIONS ..............3-1 Common Controls and Techniques ................3-1 Windows Controls ......................3-2 Pointing Device Cursor .................... 3-2 Command Button ....................
- Page 8 CAPINTEC, INC. CAPTUS ® 4000e Invalidate User ......................4-13 Revalidate User ......................4-13 Displaying Data by Date .................... 4-13 Printing Report ......................4-13 Exiting ........................4-14 FIRST TIME STARTUP ....................4-14 System Parameters ....................4-15 Quality Assurance Tests .................... 4-16 Reference Pages ....................
- Page 9 CAPINTEC, INC. CAPTUS ® 4000e Deleting Patient Records ................... 7-15 VIEWING AND CHANGING THE SETUP ..............7-16 MAKING MEASUREMENTS ..................7-18 Counting Selected Patient ..................7-20 Administering Dose ....................7-24 RESULTS ........................7-24 Patients Reports ......................7-25 Sources of Error ......................7-28 WIPE TESTS ........................
- Page 10 CAPINTEC, INC. CAPTUS ® 4000e EDITING PATIENTS ..................... 9-14 DELETING PATIENTS....................9-15 MAKING MEASUREMENTS ..................9-16 Data Acquisition ......................9-20 CREATING AN ARCHIVE ..................... 9-21 OPENING AN ARCHIVE ....................9-22 PATIENT REPORTS ....................9-23 Displaying and Printing ....................9-23 Exporting ........................9-26 BIOASSAY ........................
- Page 11 CAPINTEC, INC. CAPTUS ® 4000e Recall Counting Setup ..................... 12-14 Restore Current Settings ..................12-14 PRINTING MCA DATA ....................12-14 CUSTOMIZING MCA SETTINGS ................12-14 High Voltage ......................12-15 Gain ......................... 12-15 Zero Offset ....................... 12-16 Threshold ......................... 12-16 MANUAL CALIBRATION ..................... 12-17 ARCHIVE ..........................
- Page 12 CAPINTEC, INC. CAPTUS ® 4000e CLEANING AND MAINTENANCE ................... 15-1 INTRODUCTION ......................15-1 CLEANING and DISINFECTING ................... 15-2 Cleaning Instructions ....................15-2 Disinfecting Instructions ..................... 15-4 Liner ........................15-4 Printer ........................15-4 PREVENTIVE MAINTENANCE ..................15-4 DISPOSAL ........................15-5 SERVICING ........................15-6 Collimator Block Inspection ..................
- Page 13 CAPINTEC, INC. CAPTUS ® 4000e INDEX WARRANTY DECLARATION OF CONFORMITY TOC-8 TABLE OF CONTENTS August 20...
-
Page 14: Safety
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 1 SAFETY GENERAL ....................... 1-1 SYMBOL DEFINITIONS .................. 1-1 WARNING AND CAUTION LABELS .............. 1-2 CAUTIONS AND NOTES ................1-3 ELECTROMAGNETIC COMPATABILTIY TEST DATA ........1-7 GENERAL SAFETY TIPS ................1-11 GENERAL These warnings and instructions form an integral part of the CAPTUS ®... -
Page 15: Warning And Caution Labels
CE Mark 2017/745/EU symbol for Medical Device 2017/745/EU symbol for hazardous substance. The Captus 4000e contains lead as shielding. Waste in Electrical and Electronic Equipment (WEEE) – This symbol indicates that the waste of electrical and electronic equipment must not be disposed as unsorted municipal waste and must be collected separately. -
Page 16: Cautions And Notes
CAPINTEC, INC. CAPTUS ® 4000e CAUTION: Please reference CHAPTER 15: CLEANING AND MAINTENANCE for instructions on how to change the fuses of the Isolation Transformer. CAUTION: For continued protection, replace only with same type and rating of fuse(s). A fire hazard may exist if the wrong rating of fuse is installed. - Page 17 CAUTION: If any Computer, Printer or cables other than the model supplied by Capintec is used, the safety of the unit may be compromised or other devices located in the same general area as the CAPTUS 4000e may ®...
- Page 18 CAUTION: Only the included USB devices should be connected to the USB ports on the Computer. If any USB devices other than the models supplied by Capintec are used, the safety of the unit may be compromised or other devices located in the same general area as the CAPTUS ®...
- Page 19 Note: It is recommended that periodic (every five years) re-calibration of the unit be performed only by Capintec’s Authorized Service Center (reference CHAPTER 15: CLEANING AND MAINTENANCE) to guarantee that the instrument's high reliability is maintained).
-
Page 20: Electromagnetic Compatabiltiy Test Data
CAPINTEC, INC. CAPTUS ® 4000e ELECTROMAGNETIC COMPATABILTIY TEST DATA Table 1 - Guidance and Manufacturer's Declaration - Emissions All ME Equipment and ME Systems Emissions Test Compliance Electromagnetic Environment - Guidance RF Emissions CAPTUS® 4000e uses RF energy only for... - Page 21 CAPINTEC, INC. CAPTUS ® 4000e ±8kV Contact ±8kV Contact Floors should be IEC 61000-4-2 ±15kV Air ±15kV Air wood, concrete or ceramic tile. If floors synthetic, r/h should be at least Mains power quality ±2kV Mains ±2kV Mains should be that of a IEC 61000-4-4 ±1kV I/Os...
- Page 22 CAPINTEC, INC. CAPTUS ® 4000e Table 3 - Guidance and Manufacturer's Declaration - Immunity ME Equipment and ME Systems that are NOT Life- supporting Immunity Test IEC 60601 Test Electromagnet Compliance Level Level Environment - Guidance Portable and mobile communications...
- Page 23 CAPINTEC, INC. CAPTUS ® 4000e Interference may occur in the vicinity of equipment containing a transmitter. Table 4 - Recommended Separation Distances between portable and mobile RF Communications equipment and the CAPTUS® 4000e ME Equipment and ME Systems that are NOT Life-supporting...
-
Page 24: General Safety Tips
CAPINTEC, INC. CAPTUS ® 4000e GENERAL SAFETY TIPS • Unplug the equipment before cleaning it. Use only a damp cloth; do not use solvents or aerosol cleaners. • To protect the equipment from overheating, make sure none of the openings (vents) on the computer, monitor or the printer are blocked. -
Page 25: Functional & Technical Description
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 2 FUNCTIONAL & TECHNICAL DESCRIPTION INTENDED USE ....................2-1 INCIDENT REPORTING .................. 2-1 CONTRAINDICATION ..................2-2 OPERATOR PROFILE ..................2-2 OPERATOR TRAINING .................. 2-2 FUNCTIONAL DESCRIPTION ................ 2-2 Thyroid Uptake ................... 2-2 Wipe Test ....................2-3 Bioassay ..................... -
Page 26: Contraindication
The user can decide if contraindications from their perspective but the manufacturer (Capintec) makes the device safe for use in all patient groups. The risks with the Radioactive Iodine uptake test are minimal. -
Page 27: Wipe Test
CAPINTEC, INC. CAPTUS ® 4000e thyroid hormone secretion in ectopic tumors, hormonal release in thyroid injury, after intake of thyroid hormone, or in Jod-Basedow disease. During the treatment of thyroid cancer by I- 131 ablation, uptake measurements are used to monitor thyroid activity. -
Page 28: Detector
CAPINTEC, INC. CAPTUS ® 4000e • Cables for connecting Detector, CAP-MCA, etc. • 15-foot power cord with hospital grade plug • Windows 8.1 or later, • CAPTUS 4000e Software Installation CD including Owner’s Manual ® • Optional Well Counter with drilled Well Detector... - Page 29 CAPINTEC, INC. CAPTUS ® 4000e Electronics associated with the process include the high voltage power supply, amplifiers, and gain adjustments. The high voltage power supply provides voltage to the photomultiplier tube for the light to electrical impulse conversion and amplification process. The amplifiers linearly amplify the electrical signal produced by the photomultiplier tube before the signal is passed to the MCA.
-
Page 30: Technical Specifications
CAPINTEC, INC. CAPTUS ® 4000e TECHNICAL SPECIFICATIONS System Hardware Detectors, Data Acquisition, and Computer All-in-One Touchscreen computer system including the MS Windows operating ® system, CAP-MCA, a dual input Multi-Channel Analyzer, scintillation detector(s), a mouse or trackball pointing device, a color printer, and an easy to use CAPTUS ®... -
Page 31: Computer System (Minimum System Requirements)
CAPINTEC, INC. CAPTUS ® 4000e Computer System (Minimum System Requirements) PC compatible, Intel ® Celeron ® Processor CD-ROM Drive 500MB free Hard Drive space 4GB RAM SVGA Graphics (1600×900, 24 bit Color) 19.5” Display (Touchscreen recommended) Keyboard and Mouse / Trackball MS Windows ®... -
Page 32: Tus Probe Detector
CAPINTEC, INC. CAPTUS ® 4000e TUS Probe Detector NaI(TI) flat face crystal / PM Tube ..5.1 x 5.1 cm ... (2” x 2”) Monoline construction Resolution (Full Width-Half Max) – ≤9.5% or better for Cs137 Lead Shielded Collimator Complies with ANSI Standard N44.3-1973 Coaxial Signal/High Voltage Cable ... -
Page 33: Storage
CAPINTEC, INC. CAPTUS ® 4000e Storage The instrument should be stored where the temperature is stable and the range is from +39°F to +110°F (+4°C to +43°C) and the maximum relative humidity is 90% non-condensing for maximum reliability. The instrument should be stored where the barometric pressure is within a range of 15–33 inches of mercury (51–112 kilopascals). -
Page 34: Printer
Life Time (End of Life) The Captus 4000e Life Time (End of Life) is considered to be ten years after date of shipment. Service, support, and spare parts will be provided for this time. After ten years, the system is no longer considered “state of the art” and should be replaced. -
Page 35: General Operating Instructions
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 3 GENERAL OPERATING INSTRUCTIONS INTRODUCTION ..................... 3-1 WINDOWS OPERATING CONVENTIONS ............3-1 Common Controls and Techniques ............3-1 Windows Controls ..................3-2 CAPTUS SCREENS ..................3-7 Typical Test Screen ..................3-9 Typical Input Screen ................. 3-10 Measurement Screen ................ -
Page 36: Windows Controls
CAPINTEC, INC. CAPTUS ® 4000e Windows Controls The most common Windows controls are described below. When controls are selected, they cause functions or actions to be performed. A control can be selected in several ways; • Using the pointing device – Positioning the pointing device cursor over the control and clicking the left pointing device button selects the control. -
Page 37: Command Button
CAPINTEC, INC. CAPTUS ® 4000e Command Button The most common control in the CAPTUS ® 4000e is the command button, for example the command button. A command button can be activated as described below: • positioning the pointing device over the button and clicking the pointing device's left button once, •... -
Page 38: Text Entry Box
CAPINTEC, INC. CAPTUS ® 4000e Check Box Check boxes are also used to select or enable various options; however, check boxes work as individual controls not in groups. When a check box is true a “√” will appear in the box. -
Page 39: Scroll Bars
CAPINTEC, INC. CAPTUS ® 4000e Left Arrow ....Moves one character to the left End ......Moves to the end of the input Home ......Moves to the beginning of the input Some text input boxes provide input verification. If the information typed in the box is invalid, i.e. -
Page 40: Spin Control
CAPINTEC, INC. CAPTUS ® 4000e Spin Control The spin control is also used to adjust values. The control consists of two back to back arrows. Clicking an arrow with the pointing device causes the value under control to change in the specified direction. The spin button can only be controlled with the pointing device, your finger or stylus. -
Page 41: Keyboard Control
Setup modules (Setup), an Isotope Reference Library (Isotopes), Archived results (Archives) and contact information for Capintec, Inc. (About). To select a module, move the pointing device’s cursor over the name of the module you wish to select. A highlight box will appear around the module title. - Page 42 CAPINTEC, INC. CAPTUS ® 4000e Figure 3-5 GENERAL OPERATING INSTRUCTIONS August 20...
-
Page 43: Typical Test Screen
CAPINTEC, INC. CAPTUS ® 4000e Typical Test Screen Patient Directory Box (List of records Selected Patient in the database) (Highlighted) Buttons Check Command Box Showing Command Highlighted Button (Currently Button (Currently Patient’s Status Inaccessible/ Accessible/ Disabled) Enabled) Figure 3-6 August 20... -
Page 44: Typical Input Screen
CAPINTEC, INC. CAPTUS ® 4000e Typical Input Screen Frame around a Text Entry Box group of Radio Buttons Current selection Text Entry Box for Comments Command Button: Command Button: Command Button: Discard Changes Save Changes Add More Wipe Made and Exit... -
Page 45: Measurement Screen
CAPINTEC, INC. CAPTUS ® 4000e Measurement Screen The Measurement screen is identical in all the test procedures and is as shown in Figure 3-8. The live time elapsed while counting appears on the upper right hand corner of the screen. -
Page 46: Add Comments Screen
CAPINTEC, INC. CAPTUS ® 4000e Add Comments Screen The screen for adding comments to reports is identical to those for all the test procedures. You may type in the comments as free text using standard editing keys. Figure 3-9 The following options are available on this screen: Insert File button or press Alt+I –... -
Page 47: Shortcut Keys
CAPINTEC, INC. CAPTUS ® 4000e SHORTCUT KEYS All Screens Operation Key(s) Cycle through all items on the screen First letter of the desired record Select from a list of records in the database (Name field), when the records box is active... -
Page 48: General Setup
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 4 GENERAL SETUP INSTALLATION ....................4-1 ENVIRONMENT REQUIREMENTS ..............4-3 Operational ....................4-3 Storage ......................4-3 POWER REQUIREMENTS ................4-4 Input Power ....................4-4 Isolation Transformer Output ..............4-4 TURN ON PROCEDURE ................. 4-4 LOG ON ...................... - Page 49 CAUTION: Only the included USB devices should be connected to the USB ports on the Computer. If any USB devices other than the models supplied by Capintec are used, the safety of the unit may be compromised or other devices located in the same general area as the CAPTUS ®...
-
Page 50: Environment Requirements
CAPINTEC, INC. CAPTUS ® 4000e CAUTION: Do not connect the CAPTUS ® 4000e to a network. If the system is attached to a network, the safety of the unit may be compromised or other devices located in the same general area as the CAPTUS ®... -
Page 51: Power Requirements
CAPINTEC, INC. CAPTUS ® 4000e The instrument should be stored where the barometric pressure is within a range of 15–33 inches of mercury (51–112 kilopascals). CAUTION: If these environmental requirements are not followed, the instrument may display erroneous readings. Always store the arm in the upright position. This place the least amount of stress on the spring arm components. -
Page 52: Log On
CAPINTEC, INC. CAPTUS ® 4000e All software necessary for proper operation of your CAPTUS ® 4000e Thyroid Uptake System has been pre-installed and tested at the factory to ensure trouble-free setup. Note: To remove main power from the system, the Isolation Transformer main power cable must be unplugged from the wall socket. -
Page 53: Sign In
CAPINTEC, INC. CAPTUS ® 4000e Figure 4-3 Log On Sign In Window Sign In Users must set up by the Administrator prior to logging on to the CAPTUS ® 4000e. User Names and Passwords are case sensitive. Input the User Name and then the Password. Asterisks (*) will appear when the Password is entered. -
Page 54: User Log On
CAPINTEC, INC. CAPTUS ® 4000e For the Administrator to log on: In the User Name text box, input Admin and press the Tab key. • • In the Password text box, input the password (AlphaDrive is default) and press the Tab key. -
Page 55: Add User
CAPINTEC, INC. CAPTUS ® 4000e Figure 4-5 User Log On Sign In Window ADD USER Only the Admin can add users. From Figure 4-4 Admin Log On Sign In Window, select the Add User button. Add User – Staff Member If the user to be added is a staff member, in the “Is User a Staff Member?”... -
Page 56: Add User - Not A Staff Member
CAPINTEC, INC. CAPTUS ® 4000e Figure 4-6 Add User (Staff Member) Window Note: In order for the Staff Member List to be displayed, Staff Members must be added in ® the Bioassay module in the CAPTUS 4000e software as described in CHAPTER 11: BIOASSAY, SECTION: ADDING STAFF MEMBERS. -
Page 57: Change Password
CAPINTEC, INC. CAPTUS ® 4000e Figure 4-7 Add User (Not a Staff Member) Window Input the new User Name and then the Password. Input the Password again in the Verify Password text box. When the OK button is selected, the new user and password will be accepted and Figure 4-4 Admin Log On will re-appear. -
Page 58: Log On Report
CAPINTEC, INC. CAPTUS ® 4000e Figure 4-8 Change Password Window The current User Name is displayed and cannot be changed. Input the New Password and then re-input it again in the Verify Password text box. Select the OK button to accept the new password. -
Page 59: Displaying Selected User
CAPINTEC, INC. CAPTUS ® 4000e Figure 4-9 Log On Report Window The User, Log On Date/Time and Log Off Date/Time are displayed in reverse chronological order. When All Users are displayed, the Delete User and Invalidate User buttons are disabled (grayed-out). -
Page 60: Invalidate User
CAPINTEC, INC. CAPTUS ® 4000e Invalidate User Invalidating a user will remove the user from the list of users who may log onto the program but it will not delete the user’s log on data. To invalidate a user, select the User Name from the Drop-Down List box and List box and select the Update button. -
Page 61: Exiting
CAPINTEC, INC. CAPTUS ® 4000e To print a report of the displayed Log On Data, select the Print button. Exiting To exit the Log On Reports window and return to Log On window, select the Done button. FIRST TIME STARTUP If this is the first time the CAPTUS ®... -
Page 62: System Parameters
CAPINTEC, INC. CAPTUS ® 4000e The system parameters for the CAPTUS ® 4000e software can be altered through the Setup module. The Setup module (Figure 4-11 Setup Module Screen) standardizes units of measurement, date format and detector type for the entire system. A report header can be entered that will appear at the top of all report printouts. -
Page 63: Quality Assurance Tests
Note: Your detector setting has been selected for the specific hardware shipped with your system. Therefore, the detector default should not be changed without consulting with the technical service staff at Capintec. 4. To enter a heading for each printed report, click in the first blank box under Report Header. -
Page 64: Reference Pages
CAPINTEC, INC. CAPTUS ® 4000e Before performing any of the Quality Assurance tests, allow the system to warm up at least 30 minutes. 1. Select the Go button beside “Enter Check Source and Chi-Square Setup”. The Quality Assurance Setup window will appear. -
Page 65: Reference Pages
CAPINTEC, INC. CAPTUS ® 4000e 5. Repeat steps 1-4 for each remaining setup (Wipe Test Groups, and Bioassay). 6. Select the Exit button or press Alt+E to close the Startup Tips window. Note: If you do not want the Startup Tips window to appear the next time CAPTUS ®... - Page 66 Note: The Serial Number, together with your software version, is to be used as a reference when contacting the Capintec Service Center for information or assistance. To get the software version, select the About button on the CAPTUS ®...
-
Page 67: Quality Assurance
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 5 QUALITY ASSURANCE INTRODUCTION ..................... 5-1 Recommended Routine for CAPTUS 4000e System ......5-1 ® SETTING UP QA PARAMETERS ..............5-2 AUTO CALIBRATION ..................5-5 About Auto Calibration ................5-10 CONSTANCY TEST ..................5-11 CHI-SQUARE TEST .................. -
Page 68: Setting Up Qa Parameters
CAPINTEC, INC. CAPTUS ® 4000e Note: If you prefer to turn off the entire system every day, then allow at least 30 minutes for system warm up before performing any calibration procedures or making any measurements. 2. Schedule for performing the Quality Assurance (QA) Tests a. - Page 69 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-1 Quality Assurance Main Screen Note: The DICOM Report button will only be displayed if the valid Cap-DICOM license is entered in your system. The following tests require setting up parameters prior to performing the test: Constancy Test, Chi-Square Test and MDA Test.
- Page 70 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-2 Quality Setup Screen 2. Constancy Test Parameters – These are provided in the Cs137 Check Source frame. Enter the Calibrated Activity and Calibration Date in the appropriate text boxes marked on the Cs137 standard source.
-
Page 71: Auto Calibration
CAPINTEC, INC. CAPTUS ® 4000e • To return to the CAPTUS ® 4000e Main screen from the QA Tests screen, select the Exit button or press the Esc Key. • Rest the pointer close to each module title on Figure 5-1 Quality Assurance Main Screen to read a brief description of that function. - Page 72 The rod should stand freely and not lean to the side. • To ensure that the Cs137 source rod is consistently placed in the center of the Probe face, a Rod Source Holder may be purchased from Capintec (reference CHAPTER 15: CLEANING AND MAINTENANCE, SECTION: ACCESSORIES AND REPLACEMENT PARTS), or •...
- Page 73 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-5 8. Next, the screen appears as shown in Figure 5-6, prompting you to perform Linearity Correction using Eu152. This step, though optional, is highly recommended for fine- tuning your system. Figure 5-6 9. Select the Yes button or press the Y key to proceed with further calibration. Otherwise select the No button or press the N key to stop here.
- Page 74 Note: If the calibration is not successful, select the Manual Calibration button or press Alt+M on the Auto Calibration screen. The MCA module is started. Refer to CHAPTER 12: MCA. If this does not correct the problem, contact Capintec’s only Authorized Service Center for service.
- Page 75 Constancy Test. If no problems are found with the above items, then the efficiency of the Cs137 standard should be re-measured. If this does not correct the problem, contact Capintec’s only Authorized Service Center for service. August 20...
-
Page 76: About Auto Calibration
CAPINTEC, INC. CAPTUS ® 4000e 17. Select the Exit button or press Alt+E to exit and return to Figure 5-1 Quality Assurance Main Screen. To print current results for record keeping, click the Print button or press Alt+P on the •... -
Page 77: Constancy Test
CAPINTEC, INC. CAPTUS ® 4000e CONSTANCY TEST CAUTION: Calibration is recommended before making any measurements. Refer to the AUTO CALIBRATION section on page 5-5. This test compares the Cs137 standard source activity entered in the setup with the activity that it measures. The activity entered in the setup is decay corrected by the software. The counts measured in the Constancy test are converted to activity using efficiency values from the Isotope Library. - Page 78 CAPINTEC, INC. CAPTUS ® 4000e Note: This is a one-time procedure and should not to be repeated unless the Constancy Test is unsuccessful. a. Select the Cs137 Efficiency button at the top of the screen or press Alt+C. The screen appears as shown in Figure 5-12. Calibrated Activity and Date are already entered from the QA Setup performed above.
- Page 79 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-13 e. Select the Start Procedure button or press Alt+S to start measurements. Follow the on-screen instructions. After the efficiency is measured, select the Exit button to return the Constancy Test screen. g. For systems with two detectors, if the efficiency has never been measured on the other detector, then repeat Step 3.
- Page 80 CAPINTEC, INC. CAPTUS ® 4000e 6. Place the Cs137 source rod in the selected detector. Since the Constancy Test is a measure of reproducibility, it is important that the source be placed in exactly the same position each time the Constancy Test is performed.
-
Page 81: Chi-Square Test
4000e If no problems are found with the above items, then the efficiency of the Cs137 standard should be re-measured. If this does not correct the problem, contact Capintec’s only Authorized Service Center for service. 10. Select the Exit button or press Alt+E to exit and return to Figure 5-1 Quality Assurance Main Screen. - Page 82 CAPINTEC, INC. CAPTUS ® 4000e 1. Select the Chi-Square Test button or press Alt+H on Figure 5-1 Quality Assurance Main Screen. The screen appears as shown in Figure 5-16 Chi-Square Test Screen. Figure 5-16 Chi-Square Test Screen 2. Note that in the Select Detector frame, PROBE is selected by default. To change the detector to Well, select the WELL radio button.
- Page 83 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-17 Quality Setup Screen 4. To change the default values in Chi-Square Test frame in Setup, click in the text boxes and input the new values. Note: The Counting Time can be set between 2 and 9,999 seconds. The Repetitions can be set between 5 and 20.
- Page 84 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-19 7. Place the Cs137 source in the same position as for Auto Calibration and Constancy Test. 8. Select the OK button to proceed with the test. Otherwise, select the Cancel button to stop the procedure.
-
Page 85: Mda Test
In no problems are found with the above items, the out of range Chi-Square value may indicate a problem with the counter/timer mechanism of the system or the detector itself. Contact Capintec’s only Authorized Service Center for service. To print current results for record keeping, select the Print button or press Alt+P on •... - Page 86 CAPINTEC, INC. CAPTUS ® 4000e This test measures counts and calculates activity in the ROI for the isotope selected in the Setup. The measurement is performed with no isotope placed in the detector, which therefore gives a value for the Minimum Detectable Activity (MDA).
- Page 87 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-23 Quality Setup Screen 4. To change the Counting Time, click in the text box and input the new value. Note: The Counting Time can be set between 1 and 9,999 seconds. 5. To select another isotope, click in the Selected Isotope drop-down box and click on the desired isotope to select it.
- Page 88 CAPINTEC, INC. CAPTUS ® 4000e performed now, the program will return to Figure 5-22 MDA Test Screen after calibration is complete. Figure 5-25 9. Remove any radioactive source from the vicinity and select the OK button to proceed with the test. Else, select the Cancel button to stop the procedure.
-
Page 89: Viewing System Parameters
CAPINTEC, INC. CAPTUS ® 4000e VIEWING SYSTEM PARAMETERS This function provides access to all of the parameters used for the measurements and calculations of results in all procedures (Thyroid Uptake, Wipe Test, QA Tests etc.) provided by the CAPTUS 4000e system. -
Page 90: Viewing And Printing History
CAPINTEC, INC. CAPTUS ® 4000e Figure 5-28 3. Type an appropriate file name and select the Save button or press Alt+S. 4. To print all of the parameters, select the Print button or press Alt+P. 5. To return to Figure 5-1 Quality Assurance Main Screen, select the Exit button or press Alt+E. - Page 91 CAPINTEC, INC. CAPTUS ® 4000e Figure 5-29 2. To view all of the results of each Auto Calibration and Constancy Test performed to date on the Probe or Well detector, select the appropriate radio button for the Probe History or Well History.
- Page 92 CAPINTEC, INC. CAPTUS ® 4000e appears as shown in Figure 5-30. Select the Yes button or press the Y key to confirm the deletion. Otherwise, select the No button or press the N key. Figure 5-30 7. To delete all of the tests on the current screen, select the Delete button or press Alt+D.
-
Page 93: Isotopes
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 6 ISOTOPES INTRODUCTION ..................... 6-1 VIEWING AND PRINTING ISOTOPE LIBRARY ..........6-2 ADDING A NEW ISOTOPE TO THE LIBRARY ..........6-3 EDITING ISOTOPE INFORMATION ............... 6-4 DELETING AN ISOTOPE ................6-5 MEASURING EFFICIENCY ................6-5 ENTERING EFFICIENCY ................ -
Page 94: Viewing And Printing Isotope Library
CAPINTEC, INC. CAPTUS ® 4000e Figure 6-1 Isotopes Main Screen The Isotope Library box lists the isotopes alphabetically, with their respective Half-Lives in minutes (M), hours (H), days (D) or years (Y), keV (for major energy peaks), and Well and Probe efficiencies, if known. -
Page 95: Adding A New Isotope To The Library
CAPINTEC, INC. CAPTUS ® 4000e ADDING A NEW ISOTOPE TO THE LIBRARY Note: All fields in bold are required before the Done button will be enabled. 1. Select the Add button on Figure 6-1 Isotopes Main Screen. Figure 6-2 will appear. -
Page 96: Editing Isotope Information
CAPINTEC, INC. CAPTUS ® 4000e 3. Click in the text boxes under Energy Information and input the peak energy values in keV. At least one peak value (Peak 1) must be input. (Input must be between 0.01 and 2046.) 4. To save the added information and return to the Isotopes Main screen, select the Done button or press Alt+D. -
Page 97: Deleting An Isotope
CAPINTEC, INC. CAPTUS ® 4000e • To locate an isotope, use the scroll bar, Page Down, Page Up, and arrow keys or press the first letter of the isotope to bring it into view. DELETING AN ISOTOPE Note: Only user added isotopes can be deleted. Isotopes in the system’s pre-existing Isotope Library cannot be deleted. - Page 98 CAPINTEC, INC. CAPTUS ® 4000e Figure 6-6 4. Click in the text box or use the Tab key to move around. Input the Calibration Activity, Calibration Date and Calibration Time from the source label and the desired Counting Time in seconds.
- Page 99 CAPINTEC, INC. CAPTUS ® 4000e Figure 6-8 7. Select the Start Procedure button or press Alt+S to start measurements. Alternatively, select the Exit button or press Alt+E to return to Figure 6-1 Isotopes Main Screen. 8. When the procedure is started, the Background measurement message window shown in Figure 6-9 will appear.
- Page 100 CAPINTEC, INC. CAPTUS ® 4000e Figure 6-10 9. After accepting the Background measurement, the message window shown in Figure 6-11 will appear, prompting you to measure the isotope selected for efficiency measurement. Figure 6-11 To measure the isotope, place the isotope standard in the Probe or Well as the message indicates and select the OK button.
-
Page 101: Entering Efficiency
CAPINTEC, INC. CAPTUS ® 4000e To end the procedure, select the Yes button or press the Y key. The screen appears as shown in Figure 6-8. Select the Start Procedure button or press Alt+S to start again or select the Exit button to quit. - Page 102 CAPINTEC, INC. CAPTUS ® 4000e 3. Select the Enter Efficiency button or press Alt+E. The window shown in Figure 6-14 will appear displaying the selected isotope’s name at the top. Efficiency values for Well and Probe may or may not already exist.
-
Page 103: Thyroid Uptake
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 7 THYROID UPTAKE INTRODUCTION ..................... 7-1 QUICK STEP GUIDE FOR THYROID UPTAKE PROCEDURE ...... 7-3 MEASUREMENT PROTOCOLS ..............7-3 Counting Methods ..................7-3 Dose Administration .................. 7-4 Adding Protocols ..................7-5 Editing Protocols ..................7-7 Deleting Protocols .................. - Page 104 CAPINTEC, INC. CAPTUS ® 4000e thyroid hormone secretion in ectopic tumors, hormonal release in thyroid injury, after intake of thyroid hormone, or Jod-Basedow disease. During the treatment of thyroid cancer by I-131 ablation, uptake measurements are used to monitor thyroid activity.
-
Page 105: Quick Step Guide For Thyroid Uptake Procedure
CAPINTEC, INC. CAPTUS ® 4000e Note: At least one measurement protocol must be set up before a patient can be added to the Patient Directory or any other function can be accessed from Figure 7-1 Thyroid Uptake Main Screen. QUICK STEP GUIDE FOR THYROID UPTAKE PROCEDURE 1. -
Page 106: Dose Administration
CAPINTEC, INC. CAPTUS ® 4000e • Measure the patient for uptakes at the required intervals. Sometimes, individual capsules may vary in activity from one to another. This method eliminates any error caused by a difference in activity between the capsule administered to the patient and a second different capsule used as a standard. -
Page 107: Adding Protocols
CAPINTEC, INC. CAPTUS ® 4000e Multiply by multiplies that count rate by appropriate factor Factor (entered when the patient is added to the database) to calculate the total activity for the entire dose given. Measure background and liquid. Then dose is Measure administered. - Page 108 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-3 Protocol Setup Window 2. Input a descriptive name for the protocol being defined in the Protocol Name text box. Spaces, numbers and other characters may be used – up to 32 characters (e.g. “Decay Correct Single Dose-1”).
-
Page 109: Editing Protocols
CAPINTEC, INC. CAPTUS ® 4000e subsequent I-131 thyroid uptake performed in order to evaluate the efficacy of the treatment. 8. Select the appropriate radio button in the Repetitions frame to select the number of measurement repetitions to be performed each time a capsule or patient is counted (the Background for the capsule is only counted once). - Page 110 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-4 2. Select the Edit button to edit any settings. Figure 7-5 will appear populated with the selected Protocol’s settings. Figure 7-5 3. Select the appropriate radio button(s) to make new selections. To change the name of the protocol, click in the Protocol Name text box and type over the existing name.
-
Page 111: Deleting Protocols
CAPINTEC, INC. CAPTUS ® 4000e 4. Select the Done button to save the changes or select the Cancel button to cancel the changes and return to Figure 7-2 Protocols Screen. • To move between boxes, use the Tab key and to move within boxes, use the or ... -
Page 112: Printing Protocol Report
CAPINTEC, INC. CAPTUS ® 4000e Figure 7-7 3. Select the Yes button or press the Y key to confirm the deletion. Otherwise, select the No button or press the N key. 4. To delete all of the protocols at once, select the Delete All button. The message window appears as shown in Figure 7-8 prompting you to confirm the deletion. - Page 113 CAPINTEC, INC. CAPTUS ® 4000e 1. On Figure 7-1 Thyroid Uptake Main Screen, select the Add button. The Patient Information screen will appear as shown in Figure 7-9, with information to be entered in three sections. Figure 7-9 • Click in the text box or use the Tab key to move from one text box to another. To select from the drop-down list, use the ...
- Page 114 CAPINTEC, INC. CAPTUS ® 4000e Dose Measured is Dose Administered (Single Dose) Measure Each Dose and Add Activity (Multiple Doses) Note: Manually add the activity of all capsules and enter the total activity in the “Total Activity” input box. Also, each capsule must have the same activity, calibration date and time.
-
Page 115: Viewing Patient Information
CAPINTEC, INC. CAPTUS ® 4000e Note: The fields in the Dosage Information section are available only when you have selected a protocol for the patient. 4. Enter General Information. Click in the Probe Distance text box and input the distance of the Probe from the •... - Page 116 CAPINTEC, INC. CAPTUS ® 4000e available. To reach a particular patient, press the first letter of the patient name. Press the arrow key if necessary, to go further down (Figure 7-10). Figure 7-10 2. Selecting a patient with partially entered data will prompt you with the screen below (Figure 7-11).
-
Page 117: Deleting Patient Records
CAPINTEC, INC. CAPTUS ® 4000e Figure 7-12 • Click in the text box or use the Tab key to move from one text box to another. To select from drop-down list, use the or arrow keys. 4. Click in the text box to be edited and type over the existing information. -
Page 118: Viewing And Changing The Setup
CAPINTEC, INC. CAPTUS ® 4000e Figure 7-13 2. Select the Delete button. The message window shown in Figure 7-14 will appear. Figure 7-14 3. Select the Yes button or press the Y key to confirm the deletion. Otherwise, select the No button or press the N key. - Page 119 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-15 Normal Range frame – This defines the Minimum and Maximum for a Normal Range of uptake percentage values measured at various Uptake Times. A percentage uptake measured below or above this range is flagged as out of range in the patient reports.
-
Page 120: Making Measurements
CAPINTEC, INC. CAPTUS ® 4000e uptake measurement results. Large (10 point) is the default font size. Medium is equivalent to 8 point; Small is equivalent to 7 point. 2. To edit values, click in the desired text box and then type in the new number. To change the Report Font Size, select the desired radio button. - Page 121 CAPINTEC, INC. CAPTUS ® 4000e • Measure Room Background. If Measure Each Dose and Add Activity is selected, then measure the • capsule (multiple capsules) in the phantom. ii. Select the Administer Dose button or press Alt+A on Figure 7-1 Thyroid Uptake Main Screen.
-
Page 122: Counting Selected Patient
CAPINTEC, INC. CAPTUS ® 4000e Counting Selected Patient 1. Select the Count Patient Dose button from Figure 7-1 Thyroid Uptake Main Screen. The screen will appear as shown in Figure 7-16. Figure 7-16 Note: If calibration has not been performed for the current day, you will be prompted with the message window shown in Figure 7-17. - Page 123 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-18 Note: Pre-Dose measurements may be skipped, if desired. This allows the Dose Measurement to be performed before the Pre-Dose measurement. You will be asked again to measure the Pre-Dose at the beginning of the Dose Administration phase of the protocol.
- Page 124 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-20 Note: If the number of Repetitions is set to 2 in the protocol, you will be prompted to repeat each measurement twice (except for the capsule Background). You may skip the second repetition, if desired.
- Page 125 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-22 A message window then appears similar to that shown in Figure 7-23 (depending upon the protocol and the stage of uptake procedure). Select the Yes button or press Y to confirm abortion of the cycle. Data collected is discarded and the program returns to the Measurement screen shown in Figure 7-16.
-
Page 126: Administering Dose
CAPINTEC, INC. CAPTUS ® 4000e Administering Dose 1. Select the Administer Dose button or press Alt+A from Figure 7-1 Thyroid Uptake Main Screen. The window appears as shown in Figure 7-25. Figure 7-25 2. If the dose is to be administered now, select the Administer Now button or press Alt+A and the current date and time from the system will be automatically input. -
Page 127: Patients Reports
CAPINTEC, INC. CAPTUS ® 4000e When a Pre-Dose measurement has been recorded, then P = Patient Neck count rate – Patient Background count rate T = Pre-Dose Neck count rate – Pre-Dose Patient Background count rate Patients Reports 1. To view or print a Patient Report, first select a patient by using the and arrow keys or Page Up and Page Down keys to move up or down in the patient list. - Page 128 CAPINTEC, INC. CAPTUS ® 4000e Using the scroll bars, scroll the report screen down to view the complete report. As shown in Figure 7-27, the results appear for each uptake time, depending on the protocol used. In this particular example, the background counts and the counts measured for the capsule are shown.
- Page 129 CAPINTEC, INC. CAPTUS ® 4000e Figure 7-28 5. To print the report, select the Print button or press Alt+P. 6. To add comments to the report, select the Enter Comment button or press Alt+C. The Comment window appears as shown in Figure 7-29. Refer to CHAPTER 3: GENERAL OPERATING INSTRUCTIONS;...
-
Page 130: Sources Of Error
Manual to assure that the instrument is working properly before each clinical test. The accuracy and measurement limits of the Captus 4000e are defined as test limits in the Quality Assurance Chapter. Overall accuracy of the thyroid uptake test depends upon many other variables including the listed sources of errors. -
Page 131: Wipe Tests
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 8 WIPE TESTS INTRODUCTION ..................... 8-1 QUICK STEP GUIDE FOR WIPE TEST ............8-3 SETTING UP DEFAULTS ................8-3 SELECTING ISOTOPES FOR WIPE TEST ............. 8-4 ADDING WIPE LOCATIONS TO PRESET GROUPS ........8-6 ADDING WIPE LOCATIONS TO USER DEFINED GROUPS ...... - Page 132 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-1 Main Screen Figure 8-2 Wipe Test Main Screen will appear. Figure 8-2 Wipe Test Main Screen WIPE TESTS August 20...
-
Page 133: Quick Step Guide For Wipe Test
CAPINTEC, INC. CAPTUS ® 4000e The Wipe Locations box shows a history of the tests performed to date and will be empty if no wipes have been performed previously. Each record appears with the location of testing, trigger level, counting time used in its measurement and the wipe type (location) (page 8-3). -
Page 134: Selecting Isotopes For Wipe Test
CAPINTEC, INC. CAPTUS ® 4000e Each group has default values set for Trigger Level (threshold activity) and Counting Time (live time duration for count collection). All wipe locations belong to one of the above groups, which automatically sets the Trigger Level and Counting Time for the wipe location. These values are also set to a default for background, which is common for all the wipe locations. - Page 135 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-4 Isotope Library Screen 2. To select the isotopes to be detected in the Wipe Test, click in the check box to the left of the isotope name in the Isotope Library box on Figure 8-4 as shown. A check should appear in the check box to indicate the isotope has been selected.
-
Page 136: Adding Wipe Locations To Preset Groups
CAPINTEC, INC. CAPTUS ® 4000e ADDING WIPE LOCATIONS TO PRESET GROUPS Note: All fields in bold are required before the Done button becomes enabled. 1. Select the Add button on Figure 8-2 Wipe Test Main Screen. Figure 8-5 Add Wipe Location Screen will appear. -
Page 137: Adding Wipe Locations To User Defined Groups
CAPINTEC, INC. CAPTUS ® 4000e • To move between data fields, use the Tab key and to select Type of wipe location, use the or arrow keys. • To better organize your wipe test routine, sort the added locations into groups depending on their functionality. -
Page 138: Editing Wipe Location Information
CAPINTEC, INC. CAPTUS ® 4000e b. Select the Add to Group button or press Alt+A. 4. To delete wipe locations in the selected group, a. Select locations in the Selected Wipes box. Note: To select a range of locations, click on the first location. Then, hold down the Shift key while clicking on the last location. - Page 139 CAPINTEC, INC. CAPTUS ® 4000e locations entered with Type as “Work Area”. Selecting Group 2 will show all locations that have been added to Group 2. Selecting All will show all the existing wipe locations in all groups. 2. Click inside the Wipe Locations box to make it active.
-
Page 140: Deleting Wipe Test Location
CAPINTEC, INC. CAPTUS ® 4000e Figure 8-9 Edit Wipe Location Screen 5. Click in the appropriate text box or select the Type frame radio button. Type over the existing information. Note: To move between data fields, use the Tab key. To select Type of wipe location, use the arrow keys. - Page 141 CAPINTEC, INC. CAPTUS ® 4000e location, click in the check box again or double-click anywhere in the grid while the desired location is highlighted and the check will be removed from the check box. Note: The status of a check box can be toggled by quickly double-clicking the desired item.
-
Page 142: Making Measurements
CAPINTEC, INC. CAPTUS ® 4000e Select All in Preset Groups to be able to see all wipe locations. • To check all wipe locations, select the Check All button or press Alt+A. • To deselect all wipe locations, select the Check None button or press Alt+L. - Page 143 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-13 Wipe Test Main Screen 4. To begin the measurements, select the Count button or press Alt+C. Figure 8-15 Wipe Test Measurement Screen will appear. Note: If calibration has not been performed for the current day, you will be prompted with the message window as shown in Figure 8-14.
- Page 144 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-15 Wipe Test Measurement Screen 5. Select the Background button or press Alt+B to measure Background counts. The message window shown in Figure 8-16 will appear. Note: Before counts can be measured on any wipe location, Background must be measured every day at least once.
- Page 145 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-17 To stop the counting midway and begin counting again, select the Re-Measure button or press Alt+R. To stop the counting midway and save the collected data and move to the next step, select the Stop and Save button or press Alt+S.
- Page 146 CAPINTEC, INC. CAPTUS ® 4000e 7. Once the Background measurement is completed and accepted, the spectrum and the Background total count are displayed as shown in Figure 8-19. Figure 8-19 8. To begin counting the wipe location, select the Count button or press Alt+C. The message window shown in Figure 8-20 will appear (the location Incoming Package is shown in the example).
-
Page 147: Results
CAPINTEC, INC. CAPTUS ® 4000e Figure 8-21 11. To add comments about the counts measured, select the Comment button or press Alt+O. 12. To print the results, select the Print button or press Alt+P. 13. If multiple wipes were selected, select the Next button to count the next wipe or press Alt+N. -
Page 148: Viewing And Printing Results
CAPINTEC, INC. CAPTUS ® 4000e The wipe test results will automatically be saved to the database. An example result screen is shown in Figure 8-22. The red HIGH shown against certain values is displayed when the net counts exceed the Trigger Level value in Setup. -
Page 149: Search
CAPINTEC, INC. CAPTUS ® 4000e The Wipe Test Search window allows users to search for certain wipe test results, export selected wipes in Excel format and print selected wipes to the system printer. The Summary Reports button allows printing of selected reports. - Page 150 CAPINTEC, INC. CAPTUS ® 4000e Wipe Type ..This allows the setting of the Wipe Type that the user is interested • in finding within the specified date range. The Wipe Type is selected by clicking the drop-down arrow and choosing the desired Wipe Type from the drop-down list.
-
Page 151: Viewing Wipe Test Data Selectively
CAPINTEC, INC. CAPTUS ® 4000e Figure 8-29 Wipe Test Search Window with Results The Wipe Test Search Results box displays the Wipe Location, Wipe Date/Time, Wipe Type, Net Count Rate and Total Activity. By default, searched results are sorted by the Wipe Date/Time, oldest one first. -
Page 152: Selecting Results
CAPINTEC, INC. CAPTUS ® 4000e Figure 8-30 Selected Wipe Test Details Window Select the Print button to print the details to the system printer or select the Done button to return to Figure 8-29 Wipe Test Search Window with Results. -
Page 153: Printing Wipe Test Results
CAPINTEC, INC. CAPTUS ® 4000e Note: It is recommended that the Save as type: is not changed from CSV Files (*.csv) Select the result or results to be exported as described above. Select the Export Selected button to export the selected result(s). Figure 8-31 Export Wipe Test Dialog Window will appear. -
Page 154: Summary Reports
CAPINTEC, INC. CAPTUS ® 4000e Summary Reports To access the Summary Reports module, select the Summary Reports menu bar button on Figure 8-32 Wipe Test Search Window. Figure 8-32 Wipe Test Search Window Figure 8-33 Wipe Test Summary Reports Window will appear. - Page 155 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-33 Wipe Test Summary Reports Window Wipes Test Summary Reports can be displayed by using the specified criteria as follows: Wipe Date/To ..This allows the setting of a date or a range of dates that the user is •...
- Page 156 CAPINTEC, INC. CAPTUS ® 4000e Location ..... This allows the setting of the Wipe Location that the user is • interested in finding within the specified date range. The Location is selected by clicking the drop-down arrow and choosing the desired location from the drop-down list.
- Page 157 CAPINTEC, INC. CAPTUS ® 4000e Figure 8-37 Summary Report – Tests By Location 1 Figure 8-38 Summary Report – Tests By Location 2 Tests By Wipe Type • Selecting this button will display a list showing the number of tests by Wipe Type for the set criteria.
- Page 158 CAPINTEC, INC. CAPTUS ® 4000e versus the overall total number of tests performed. Reference Figure 8-39 Summary Report – Tests By Wipe Type. Figure 8-39 Summary Report – Tests By Wipe Type Activity • Selecting this button will display a list showing an Activity Summary for the set criteria.
-
Page 159: Printing Wipe Test Summary Report
CAPINTEC, INC. CAPTUS ® 4000e Figure 8-40 Summary Report – Activity If data are available for the set criteria, the results will be displayed in the Wipe Test Summary Reports box. If no data are available for the selected criteria, the following... -
Page 160: Exiting Summary Reports
CAPINTEC, INC. CAPTUS ® 4000e Exiting Summary Reports Select the Done button on Figure 8-33 Wipe Test Summary Reports Window to return to Figure 8-23 Wipe Test Search Window. ISOTOPE EFFICIENCY Note: A Full Spectrum Efficiency value must be entered in the setup at all times. - Page 161 CAPINTEC, INC. CAPTUS ® 4000e Note that the Isotope Library measures peak efficiency using only the counts recorded in the ROI that surrounds the primary photopeak, and does not use total counts. This allows the peak finding program to better determine the activity of an isotope when multiple peaks or isotopes are present.
-
Page 162: Absorbed Dose Alert
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 9 ABSORBED DOSE ALERT INTRODUCTION ..................... 9-1 Basis for Conversion Calculations ............9-2 MAIN SCREEN ....................9-2 INCIDENT SETUP ................... 9-4 Setup Incident Parameters ................ 9-4 New Incident ....................9-6 Select Incident .................... 9-6 Delete Incident .................... -
Page 163: Basis For Conversion Calculations
CAPINTEC, INC. CAPTUS ® 4000e module of the CAPTUS ® 4000e software is an emergency screening module which provides an automated program to quickly measure intake and calculate absorbed organ dose in a dynamic fashion. Results which exceed predefined trigger levels are flagged to alert public health officials, who will determine the appropriate follow-up actions. - Page 164 Description. The Patient Status box will show the measurement results if a measurement has been made. Figure 9-3 Absorbed Dose ALERT Main Screen To return to the CAPTUS 4000e Main Screen from here, select the Exit button or press the Esc key. August 20...
-
Page 165: Incident Setup
CAPINTEC, INC. CAPTUS ® 4000e INCIDENT SETUP Select the Incident Setup button on Figure 9-3 Absorbed Dose ALERT Main Screen. Figure 9-4 Incident Setup Screen will appear. Figure 9-4 Incident Setup Screen The New Incident, Select Incident and Edit Incident buttons will be disabled until an initial incident has been set up. -
Page 166: Select Isotope
CAPINTEC, INC. CAPTUS ® 4000e Select Isotope The Select Isotope drop-down list box contains the isotopes that can be measured. This is a required field and must be set. The isotopes are: Cs137, Co60, I131 and Ir192. Screening Level The Screening Level is an optional entry. If a measurement is greater than the specified screening level, it will be marked as “ABOVE SCREENING LEVEL”... -
Page 167: New Incident
CAPINTEC, INC. CAPTUS ® 4000e New Incident Select the Incident Setup button on Figure 9-3 Absorbed Dose ALERT Main Screen. Figure 9-5 will appear. Figure 9-5 Note: The New Incident button will only be enabled after an initial incident has been set up. - Page 168 CAPINTEC, INC. CAPTUS ® 4000e Figure 9-6 1. Select the Select Incident button. Figure 9-7 Incident Selection Screen will appear. Figure 9-7 Incident Selection Screen The Select Incident and Delete Incident buttons will be disabled until an incident is selected (highlighted) in the Incident list box.
-
Page 169: Delete Incident
CAPINTEC, INC. CAPTUS ® 4000e 2. To select an incident, click on the incident name in the list box on Figure 9-7 Incident Selection Screen. The Incident name will become highlighted and the Select Incident and Delete Incident buttons will become enabled as shown in Figure 9-8. -
Page 170: Edit Incident
CAPINTEC, INC. CAPTUS ® 4000e Figure 9-9 Select the Yes button or press the Y key to confirm the deletion. Otherwise, select the No button or press the N key. 3. If the selected incident is the currently active incident, it cannot be deleted without first setting another incident as the current incident. - Page 171 CAPINTEC, INC. CAPTUS ® 4000e Figure 9-11 The Edit Incident button will now be enabled. Note that all of the fields and frames are disabled (grayed-out). Select the Edit Incident button. The Incident Setup Screen will appear as shown in Figure 9-12.
-
Page 172: Adding Patients
CAPINTEC, INC. CAPTUS ® 4000e 1. To input new values in any frame, click the appropriate text box or radio button or use the Tab key to move from one selection to another. 2. Select the Done button to save changes or select the Cancel button to discard the changes and return to Figure 9-3 Absorbed Dose ALERT Main Screen. - Page 173 CAPINTEC, INC. CAPTUS ® 4000e Figure 9-13 Patient Data Screen • Click in the text box or use the Tab key to move from one text box to another. To select from drop-down list, use or keys. 3. Patient Information.
- Page 174 CAPINTEC, INC. CAPTUS ® 4000e is set to Becquerel Mode, the height is input in centimeters (30 to 300). This field is optional. Weight: The patient’s weight. If the system is set to Curie Mode, the weight is input •...
-
Page 175: Editing Patients
CAPINTEC, INC. CAPTUS ® 4000e 10. Tech ID: Input the ID of the technologist performing the procedure. This field is optional and can be any combination of alphanumeric characters to a maximum of 32 characters. 11. When data entry is complete, select the Done button to save the information, or select the Cancel button to discard the changes. -
Page 176: Deleting Patients
CAPINTEC, INC. CAPTUS ® 4000e Figure 9-15 3. Click in the text box to be edited and type over the existing information. Note: All fields in bold are required before you can begin the test. 4. When finished editing the patient data, select the Done button to save the entered information, or the Cancel button to discard the changes. -
Page 177: Making Measurements
CAPINTEC, INC. CAPTUS ® 4000e Figure 9-16 1. Select the Delete button. Figure 9-17 will appear. Figure 9-17 2. Select the Yes button or press the Y key to confirm the deletion or select the No button or press the N key to cancel. - Page 178 CAPINTEC, INC. CAPTUS ® 4000e Measurements can be made immediately after adding or editing that patient’s data by selecting the Count button. For faster throughput, several patients can be added to the Patient Directory and then counted using the following procedure: 1.
- Page 179 CAPINTEC, INC. CAPTUS ® 4000e 3. Background must be measured when the program is initially entered, or if the Isotope is different from the previous measurement. 4. Select the Background button or press Alt+B to measure background. Figure 9-21 will appear.
- Page 180 CAPINTEC, INC. CAPTUS ® 4000e Note: If the number of Repetitions in the setup has been set to 2, each measurement, excluding background, will be repeated twice. You may skip the second repetition, if desired, by selecting the No button as shown in Figure 9-24.
-
Page 181: Data Acquisition
CAPINTEC, INC. CAPTUS ® 4000e 9. If more than one patient was selected, the Next Patient button will be enabled. Select this button to measure the next patient. 10. Select the Exit button or press Alt+E after all the patients have been measured to return to Figure 9-3 Absorbed Dose ALERT Main Screen. -
Page 182: Creating An Archive
CAPINTEC, INC. CAPTUS ® 4000e Figure 9-28 To accept the measurements and continue with the next step, select the Yes button or press the Y key. To discard and recount the current measurement, select the No button or press the N key. -
Page 183: Opening An Archive
CAPINTEC, INC. CAPTUS ® 4000e 2. The default filename is ALERTArchive followed by the current date. The filename can be changed. When the filename is correct, select the Save button. OPENING AN ARCHIVE Reports can be viewed and printed from a previously archived database. -
Page 184: Patient Reports
CAPINTEC, INC. CAPTUS ® 4000e Figure 9-31 3. The only available function is Report. 4. To return to the current database and access all functions, select the Exit Archive button. The patient list from the current database will be displayed and full functionality will be returned. - Page 185 CAPINTEC, INC. CAPTUS ® 4000e If the Intake was greater than the entered Screening Level, the message “ABOVE SCREENING LEVEL” will be displayed. If the Intake was lower than the entered Screening Level, the message “BELOW SCREENING LEVEL” will be displayed.
- Page 186 CAPINTEC, INC. CAPTUS ® 4000e Figure 9-33 Patient Report For each measurement, the following information is displayed: • Measurement at x days: Elapsed Time in days • Time: Date/time of the measurement • Factor: Conversion factor in cpm/µCi; (in cps/Bq for Becquerel mode) •...
-
Page 187: Exporting
CAPINTEC, INC. CAPTUS ® 4000e Figure 9-34 Select the Exit button or press Alt+X to return to Figure 9-3 Absorbed Dose ALERT Main Screen. Exporting On the Absorbed Dose ALERT Main Screen, select the Report button. The screen appears as shown in Figure 9-35, displaying a summary of all the patients in the database. - Page 188 CAPINTEC, INC. CAPTUS ® 4000e Figure 9-35 The drop-down list in the Select Incident frame contains a list of all incidents in the database. Select an incident to display a summary report for that incident. Select the Export button to export the summary to an Excel file. The Export dialog will appear.
- Page 189 CAPINTEC, INC. CAPTUS ® 4000e Figure 9-36 Selecting Excel File Input a file name and select the Save button. You can then open the file in Excel and see the same data as in the summary presented in a spreadsheet.
-
Page 190: Bioassay
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 10 BIOASSAY INTRODUCTION .................... 10-1 BIOASSAY SETUP ..................10-2 I 131/I 125 and I 123/1 125 Cross Contamination ........10-9 ADDING STAFF MEMBERS ................10-9 EDITING STAFF MEMBER INFORMATION ..........10-10 DELETING STAFF MEMBER ............... 10-11 MAKING MEASUREMENTS ................. -
Page 191: Bioassay Setup
CAPINTEC, INC. CAPTUS ® 4000e Figure 10-1 Main Bioassay Screen 4000e Main screen from this screen, select the Exit button or To return to the CAPTUS ® press the Esc key. BIOASSAY SETUP Note: The Administrator must be logged into the CAPTUS ®... - Page 192 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-2 Note: No values can be entered directly into the grayed text boxes. 2. In the section Isotope Used in Bioassay, select which Isotopes are to be used for the Bioassay Test by placing a check in the corresponding check box of the appropriate isotopes (I 131, I 125 and I 123).
- Page 193 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-3 Click in the check box to select one or more isotopes for efficiency measurement (a check should appear in the box). Depending on the isotopes selected, the boxes below (Calibration Activity, Calibration Date, Calibration Time) the selected isotope become enabled.
- Page 194 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-5 iv. Select the Start Procedure button or press Alt+S to start measurements, or select the Exit button or press Alt+E to return to the Bioassay Setup screen (Figure 10-2). When the procedure is started, the program will lead you through a sequence of measurements.
- Page 195 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-7 vi. The screen shown in Figure 10-8 appears next, prompting you to measure the selected isotope for efficiency measurement. Figure 10-8 To measure the isotope, place the isotope standard in the Neck Phantom exactly simulating the geometry used in the Bioassay measurement and select the OK button.
- Page 196 CAPINTEC, INC. CAPTUS ® 4000e To abort the procedure, select the Yes button or press the Y key. Figure 10-5 will re-appear. Select the Start Procedure button to restart the measurement or the Exit button to return to the Bioassay Setup screen (Figure 10-2).
- Page 197 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-11 ii. To input the Isotope Efficiency, click in the appropriate text box (I 131, I 125 or I 123) and input the known efficiency value (0.001-100%). iii. To input the I 131/ I 125 or I 123/I 125 Cross Contamination ratios, click in the appropriate text box and input the known ratio (0.0001-1).
-
Page 198: I 131/I 125 And I 123/1 125 Cross Contamination
CAPINTEC, INC. CAPTUS ® 4000e I 131/I 125 and I 123/1 125 Cross Contamination The CAPTUS ® 4000e system, when measuring I 131 and I 123, generates counts that also appear in the I 125 ROI. This is generally referred to as “spillover” or “cross contamination.”... -
Page 199: Editing Staff Member Information
CAPINTEC, INC. CAPTUS ® 4000e Note: Each Staff Member being added must have a unique Staff ID corresponding only to that particular Staff Member. Examples would be the Staff Member’s social security number, employee number, etc. 3. When finished adding Staff Member data, select the Done button or press Alt+D to save the entered information, or the Cancel button or press Alt+C to discard the changes. -
Page 200: Deleting Staff Member
CAPINTEC, INC. CAPTUS ® 4000e DELETING STAFF MEMBER Note: Staff Members can only be deleted by the Administrator. Note: If you wish to save Staff Member information before deleting, refer to CHAPTER 13: ARCHIVE. 1. To select one or more staff members for deletion, click in the check box to the left of the staff member name in the Staff Member Directory box on the Main Bioassay screen (Figure 10-14). -
Page 201: Making Measurements
CAPINTEC, INC. CAPTUS ® 4000e Figure 10-16 5. Select the Yes button or press the Y key to confirm the deletion. Otherwise, select the No button or press the N key. MAKING MEASUREMENTS CAUTION: Calibration is recommended before making any measurements. Refer to CHAPTER 5: QUALITY ASSURANCE TESTS;... - Page 202 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-18 Note: If calibration has not been performed, you will be prompted with the screen as shown in Figure 10-19. If calibration is performed, after the calibration is complete, the program will return to the Bioassay measurement screen shown in...
- Page 203 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-20 Beginning from here, the program guides you through a sequence of measurements. Simply follow the on-screen instructions as explained below. a. Verify that there are no radioactive sources in the area and select the OK button to measure background counts.
-
Page 204: Viewing And Printing Reports
CAPINTEC, INC. CAPTUS ® 4000e Figure 10-22 To begin counting, select the OK button. The spectrum is displayed as shown in Figure 10-21 above. To cancel measurements, select the Cancel button. The screen then appears as shown in Figure 10-23. - Page 205 CAPINTEC, INC. CAPTUS ® 4000e 3. The top section displays the Staff Directory box with the previously selected Staff Member highlighted and the Staff Member’s report in the lower section. To view another Staff Member’s report, click on that Staff Member in the upper section.
- Page 206 CAPINTEC, INC. CAPTUS ® 4000e Figure 10-25 5. To print the report displayed on the screen, select the Print Staff Member button or press Alt+P. 6. To print reports for all the staff members, select the Print All button or press Alt+A.
-
Page 207: Time Activity Analysis
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 11 TIME ACTIVITY ANALYSIS INTRODUCTION .................... 11-1 SETTING UP PARAMETERS ................ 11-2 MAKING MEASUREMENTS ................11-5 Saving the Results ..................11-8 VIEWING PREVIOUSLY SAVED RESULTS ..........11-9 INTRODUCTION Time Activity Analysis (TAA) permits the user to make repeated measurements at timed intervals. -
Page 208: Setting Up Parameters
CAPINTEC, INC. CAPTUS ® 4000e Figure 11-1 Time Activity Analysis screen To return to the CAPTUS ® 4000e Main screen from the Time Activity Analysis screen, select the Exit button or press Alt+E or press the Esc key. SETTING UP PARAMETERS Note: All fields in bold are required. - Page 209 CAPINTEC, INC. CAPTUS ® 4000e Figure 11-2 a. To input parameters from a previously saved .tim2 file, select the Load From File button or press Alt+L. The screen appears as shown in Figure 11-3. Select the file name to open it. Parameters are automatically entered into the appropriate boxes.
- Page 210 CAPINTEC, INC. CAPTUS ® 4000e To enter a brief comment about the test being performed, click in the ID text box and type in the text. This field is not required and the description can be any combination of alphanumeric characters to a maximum of 40 characters.
-
Page 211: Making Measurements
CAPINTEC, INC. CAPTUS ® 4000e Figure 11-4 The default folder for saving this file is C:\Captus4000e\Data\. The setup will be saved with .tim2 extension. Type in the file name and select the Save button or press Alt+S to save. Note: It is recommended that the Save as type: is not changed from Time Activity Files (*.tim2) - Page 212 CAPINTEC, INC. CAPTUS ® 4000e Note: If calibration has not been performed, you will be prompted with the screen as shown in Figure 11-5. If calibration is performed, after the calibration is complete, the program will return to the Time Activity Analysis screen shown in Figure 11-1.
- Page 213 CAPINTEC, INC. CAPTUS ® 4000e Figure 11-7 4. To stop the counting, select the Abort button or press Alt+A. The collected data is discarded. 5. After the counting is complete, results are shown in the default Data mode. The data modes are: Raw Only –View raw data only.
-
Page 214: Saving The Results
CAPINTEC, INC. CAPTUS ® 4000e 7. To exit the Time Activity Analysis module and return to the CAPTUS ® 4000e Main screen, select the Exit button or press Alt+E. Saving the Results 1. To save the measured data, select the Save Results button or press Alt+S on the Time Activity Analysis screen. -
Page 215: Viewing Previously Saved Results
CAPINTEC, INC. CAPTUS ® 4000e Figure 11-10 The default directory for saving the file is C:\Captus4000e\Data\. Type in the file name and select the Save button or press Alt+S to save as .xls file. Note: It is recommended that the Save as type: is not changed from Time Activity Results (*.xls) - Page 216 CAPINTEC, INC. CAPTUS ® 4000e Figure 11-11 2. Select the desired file and select the Open button. The results and spectrum will appear on the Time Activity Analysis screen. 11-10 TIME ACTIVITY ANALYSIS August 20...
-
Page 217: Mca
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 12 INTRODUCTION .................... 12-1 ACQUISITION AND COUNT DATA ............... 12-2 Channel Data Display ................12-2 CHANGING DETECTORS ................12-3 SPECTRUM SCALE & ZOOMING ..............12-3 Vertical Zoom .................... 12-3 Horizontal Zoom ..................12-4 COUNTING SETUP ..................12-7 SPECTRUM ID (NAME) ................. -
Page 218: Acquisition And Count Data
CAPINTEC, INC. CAPTUS ® 4000e Select the MCA button or press Alt+M on the CAPTUS ® 4000e Main screen to access the MCA Module. Figure 12-1 MCA Main Screen will appear. Figure 12-1 MCA Main Screen ACQUISITION AND COUNT DATA The MCA Main Screen provides all controls necessary for data acquisition. -
Page 219: Changing Detectors
CAPINTEC, INC. CAPTUS ® 4000e CHANGING DETECTORS To switch detectors, simply select the desired detector from the Detector frame on the lower right side of Figure 12-1 MCA Main Screen. The program will automatically switch the signal input to the selected detector, and reset the stored calibration settings for that detector. -
Page 220: Horizontal Zoom
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-3 Horizontal Zoom The Horizontal scale can be zoomed in two ways: • Pre-defined channel ranges, or • User selectable range Pre-Defined Channel Ranges There are four pre-defined channel ranges built into the CAPTUS ®... -
Page 221: User Selectable Range
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-4 User Selectable Range The user can select any area within the spectral display area to zoom. To expand the horizontal scale to a particular area, right-click in the spectral display area as follows (Refer to Figure 12-5): 1. - Page 222 CAPINTEC, INC. CAPTUS ® 4000e Right-click on left side of range Right-click on right side of range Figure 12-5 3. To restore the horizontal scale to the default value (0 to 1023), right-click anywhere in the spectral display area as shown in Figure 12-6.
-
Page 223: Counting Setup
CAPINTEC, INC. CAPTUS ® 4000e COUNTING SETUP The acquisition may be automatically stopped by preset live time, real time, or peak counts. After selecting the Counting Setup button on the menu bar, the screen illustrated in Figure 12-7 will appear. -
Page 224: Regions Of Interest (Roi)
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-8 Type the identifying information into the text box. Select the Done button or press Alt+D to save the ID or select the Cancel button or press Alt+C to cancel the ID and return to Figure 12-1 MCA Main Screen. - Page 225 CAPINTEC, INC. CAPTUS ® 4000e Define an ROI by typing – Type in the lowest channel of the ROI in the Start • Channel text box, and the highest channel in the End Channel text box. Define an ROI by clicking in the spectral display area – Place the arrow on the left •...
-
Page 226: Save And Restore Count Data Or Mca Settings
CAPINTEC, INC. CAPTUS ® 4000e SAVE AND RESTORE COUNT DATA OR MCA SETTINGS Spectral count data and MCA settings may be saved and recalled for future evaluation and use. Note: Saving a spectrum stores the spectrum and the MCA settings. Saving MCA settings stores only the settings and not the spectrum. -
Page 227: Recall Spectrum
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-13 Input the desired file name for the spectrum. The default folder will be C:\Captus4000e\Data, with a .mca file name extension. To save the data to another folder, browse to the desired location, then input the file name. To save the spectrum, select the Save button. -
Page 228: Export To Excel
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-14 Select the desired spectrum from the list. The default folder is C:\Captus4000e\Data. To load a spectrum from a different folder, browse to the desired location, and then select the file name. To load the spectrum, select the Open button. -
Page 229: Export Raw Spectrum
CAPINTEC, INC. CAPTUS ® 4000e Export Raw Spectrum The MCA raw spectrum count data can be exported to Excel format. This includes counts in all channels (it does not include the active ROIs). To export the raw spectrum data, select Export Raw Spectrum from the Save Results button on the menu bar. -
Page 230: Recall Counting Setup
CAPINTEC, INC. CAPTUS ® 4000e Note: It is recommended that the Save as type: is not changed from Counting Setup Files (*.csu) The MCA settings stored in the file will be the Counting Method, Counting Time/Counts and defined ROIs. Recall Counting Setup To retrieve stored counting setup, select Load Previous Counting Setup from the Load Previous button on the menu bar. -
Page 231: High Voltage
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-16 High Voltage The voltage level may be changed to a value between 0 and 1070 Volts. Use the scroll bar below the High Voltage indicator to adjust the value. Either click the right (+) and left (-) arrows, or drag the scroll bar. -
Page 232: Zero Offset
CAPINTEC, INC. CAPTUS ® 4000e Figure 12-17 Gain is adjusted by typing the desired gain into in one or both of the Gain text boxes (Gain range is 1 to 256). In order to activate the newly entered gain, select the Done button or press Alt+D to return to Figure 12-16. -
Page 233: Manual Calibration
CAPINTEC, INC. CAPTUS ® 4000e To provide a different energy calibration ratio, use the Cesium 137 rod source to • provide two peaks of known energy. Use the Gain control to adjust the location of the 662 keV peak to a desired channel. Then use the Zero offset adjust controls to position the 32 keV peak into a second desired channel. - Page 234 CAPINTEC, INC. CAPTUS ® 4000e Select the Save button to store the calibrated settings for all modules of the CAPTUS ® 4000e system. Settings will be saved for the selected detector only. Note: Once the calibration settings are saved in the MCA for Manual Calibration Screen, these will serve as a baseline for Autocalibration, which can be reattempted if it failed initially.
-
Page 235: Archive
CAPINTEC, INC. CAPTUS ® 4000e CHAPTER 13 ARCHIVE INTRODUCTION .................... 13-1 DOING SYSTEM BACKUP (CREATING AN ARCHIVE)........ 13-3 OPENING AN ARCHIVE ................13-4 VIEWING AND PRINTING RECORDS FROM ARCHIVE ....... 13-5 Selecting Tests for Viewing ..............13-5 Viewing or Printing a Record ..............13-6 Printing All Records in Selected Tests ............ - Page 236 CAPINTEC, INC. CAPTUS ® 4000e Figure 13-1 Main Archive Screen The empty box in the middle of the screen will display saved data when an archive file is opened. Each record appears with the name of the test performed, name and ID (if applicable) of the subject.
-
Page 237: Doing System Backup (Creating An Archive)
CAPINTEC, INC. CAPTUS ® 4000e DOING SYSTEM BACKUP (CREATING AN ARCHIVE) 1. Select the Create Archive button. The screen appears as shown in Figure 13-2. Figure 13-2 2. The default file name appears in the File name box as ArchiveMMDDYYYY (if the default for date format in system setup is MMDDYYYY), where MMDDYYYY is the current date or the date of archive creation. -
Page 238: Opening An Archive
CAPINTEC, INC. CAPTUS ® 4000e OPENING AN ARCHIVE 1. To open an archive, select the Open Archive button. The screen appears as shown in Figure 13-3. Figure 13-3 2. The screen shows existing archive files in the default C:\Captus4000e\Archive folder. -
Page 239: Viewing And Printing Records From Archive
CAPINTEC, INC. CAPTUS ® 4000e VIEWING AND PRINTING RECORDS FROM ARCHIVE Selecting Tests for Viewing Once an archive is opened, the records stored there are listed in the box on the middle of the Archive screen as shown below in Figure 13-4. The name of the archive file appears at the top of the box. -
Page 240: Viewing Or Printing A Record
CAPINTEC, INC. CAPTUS ® 4000e Figure 13-5 3. To select all the tests at once, select the Check All button or press Alt+A. Clicking in the check boxes again, will uncheck or deselect the test. Viewing or Printing a Record 1. - Page 241 CAPINTEC, INC. CAPTUS ® 4000e 4. Select the View Selection button to view a detailed report of the selected record as in Figure 13-7. All information on the subject, collected counts data and calculated results appear. Figure 13-7 5. To save the report displayed on the screen to another file, select the Save to File button or press Alt+S.
-
Page 242: Printing All Records In Selected Tests
CAPINTEC, INC. CAPTUS ® 4000e • For a quick print of the selection without viewing the detailed report on the screen, select the Print Selection button on the left of Figure 13-1 Main Archive Screen. • To print detailed reports of all the listed records on the current Archive screen, select the Print All Listed button. -
Page 243: Custom Protocols
Integration with Excel allows the analysis of data and reporting to be at any level of complexity – and completely user defined. Capintec provides a sophisticated Excel Workbook that handles importing all the data from the protocol automatically. There are worksheets in the workbook that are used for data access, user-defined data analysis (formulas are entered on these sheets), and reporting. -
Page 244: Starting Custom Protocols
CAPINTEC, INC. CAPTUS ® 4000e daily use to avoid confusion and/or alteration by the technologist. A report workbook takes the data from the analysis sheet, and prints a customized report for the study that can be included in the patient record. The initial configuration of the workbook requires some basic knowledge of Microsoft Excel (such as creating formulas, linking data from one worksheet to another, etc.). -
Page 245: Protocols
CAPINTEC, INC. CAPTUS ® 4000e PROTOCOLS To Add, View, or Delete Protocols, select the Protocols button on Figure 14-1 Custom Protocols Main Screen. Figure 14-2 Main Protocol Screen will appear. Figure 14-2 Main Protocol Screen Adding Protocols 1. Select the Add button on Figure 14-2 Main Protocol Screen. The Protocol Setup screen will appear as shown in Figure 16-3. - Page 246 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-3 2. Enter a descriptive name for the protocol being defined in the Protocol Name text box. (32 characters max) 3. Input the number of measurements that will be made for the protocol in the Number of Measurements text box.
- Page 247 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-4 8. Up to 5 additional fields may be entered. These are all numeric fields. Input a name (32 characters max) for each field that will be needed. Fields that are named will be available when a patient is entered.
- Page 248 CAPINTEC, INC. CAPTUS ® 4000e 11. In the Select Isotope drop-down list box, select the Isotope that will be measured. The ROIs appropriate for the selected detector and isotope will be filled in. These ROIs may be edited or added to.
-
Page 249: Viewing Protocols
CAPINTEC, INC. CAPTUS ® 4000e 19. If special instructions are to be displayed for the measurement, type the instructions in the Instructions for Sample text box. 20. When all the fields are entered, select the Next button or press ALT+N to enter data for the next measurement. -
Page 250: Patient Data
CAPINTEC, INC. CAPTUS ® 4000e To enter or change the name of the workbook to be used to analyze the data for the protocol, from Figure 14-2 Main Protocol Screen, select the Excel File button. If the selected workbook has already been assigned to a protocol, an error message will appear. - Page 251 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-8 5. Click in the field box or use Tab key to move from one field box to another. 6. Input the Patient Information. In the Sex drop-down list box, select from the list or press the M or F key.
-
Page 252: Editing Patient Information
CAPINTEC, INC. CAPTUS ® 4000e Figure 14-9 9. In the Comment text box, enter a comment if desired. 10. To save the data, select the Done button or press ALT+D. To discard the data and return to Figure 14-1 Custom Protocols Main Screen, select the Cancel button or press ALT+C. -
Page 253: Deleting All Patients
CAPINTEC, INC. CAPTUS ® 4000e Note: The status of a check box can be toggled by quickly double-clicking the desired item. Figure 14-10 1. Select the Delete button on Figure 14-1 Custom Protocols Main Screen. Figure 14-11 will appear. Figure 14-11 2. -
Page 254: Making Measurements
CAPINTEC, INC. CAPTUS ® 4000e MAKING MEASUREMENTS CAUTION: Before making any measurements, calibrate the instrument for more accurate results. Calibration is recommended every day. Refer to CHAPTER 5: QUALITY ASSURANCE, for the procedure. 1. To select a patient for the test, click on the desired record in the Patient Directory list box Figure 14-1 Custom Protocols Main Screen. -
Page 255: Data Acquisition
CAPINTEC, INC. CAPTUS ® 4000e 3. If one or more options were checked for the current measurement, the Measurement Input Screen will be shown as in Figure 14-15. In the example, both Actual Sample Volume and Sample Date and Time have been selected for Measurement Number 1. - Page 256 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-16 To stop the counting midway and begin counting again, select the Re-Measure button or press Alt+R. To stop the counting midway and save collected data and move to the next step, select the Stop and Save button or press Alt+S.
-
Page 257: After Counting
CAPINTEC, INC. CAPTUS ® 4000e After Counting After the measurement has been made, a summary of the results is shown. In the example below (Figure 14-18), 2 ROIs were selected and the measurement was background. Figure 14-18 Select the Print button or press ALT+P to print the spectrum and the summary. -
Page 258: Custom Protocols Excel Workbook
CAPINTEC, INC. CAPTUS ® 4000e CUSTOM PROTOCOLS EXCEL WORKBOOK An Excel Workbook must be set up for each protocol. The protocol must first be set up in the CAPTUS ® 4000e program. In order to accurately create formulas for the protocol, a dummy patient should be entered and all the measurements simulated (e.g. - Page 259 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-20 Note: If the Security Warning message appears after opening the Custom Protocol workbook, select the Enable Content button to enable the functionality of the workbook. From the Custom Protocol Workbook Splash Screen (Figure 14-20), select the Custom Protocol Workbook Setup button (Figure 14-25).
-
Page 260: Setting Up The Workbook
CAPINTEC, INC. CAPTUS ® 4000e Figure 14-22 Select the OK button. The message box shown in Figure 14-23 will appear. Figure 14-23 You may continue the Setup procedure now or exit and return later. If you choose to exit now and continue the Setup procedure later, when you re-open the CustomMeasurement workbook, the message box in Figure 14-24 will appear. -
Page 261: Protocol Selection
CAPINTEC, INC. CAPTUS ® 4000e Figure 14-25 Protocol Selection The Protocol Selection Sheet (Figure 14-26) will appear. Figure 14-26 Input the Protocol Index for the desired Protocol in the indicated box and press the ENTER key. The message in Figure 14-27 will appear. -
Page 262: Patient Selection
CAPINTEC, INC. CAPTUS ® 4000e Figure 14-28 Select the OK button and then select the Protocol Data tab on the bottom of the page. The Protocol Data sheet is opened and the details of the protocol will then be shown (Figure 14-29). This sheet is for reference only. - Page 263 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-30 Selecting the Yes button will allow you to continue with the Setup and selecting the No button will return you to the Protocol Data sheet (Figure 14-29). If there are patients associated with the protocol, a list of the patients for the protocol will be displayed (Figure 14-31).
-
Page 264: Entering Formulas
CAPINTEC, INC. CAPTUS ® 4000e Figure 14-32 If the selected patient has no measurements then the message box shown in Figure 14-33 will be displayed. Figure 14-33 Selecting the Yes button will allow you to continue with the Setup and selecting the No button will return you to the Patient List sheet. -
Page 265: Creating A Report
CAPINTEC, INC. CAPTUS ® 4000e Figure 14-34 Calculations may be made using data from the Patient Data sheet. For example, if the count time for the first test is needed, it would be referred to as ‘Patient Data’!I10. Creating a Report To set up a report, select the Report tab at the bottom of the workbook. -
Page 266: Viewing Patients From Workbook (User Mode)
CAPINTEC, INC. CAPTUS ® 4000e In the example above, headings have been entered explicitly. Note: Make sure you save the workbook before exiting and after any changes have been made. Note: Re-entering the Excel workbook in Setup mode and entering a Protocol Index number different from the one that was previously saved with the workbook will cause the message box shown in Figure 14-36 to appear. - Page 267 CAPINTEC, INC. CAPTUS ® 4000e Figure 14-37 3. The Patient list will be displayed (Figure 14-38). Figure 14-38 4. Click on any cell of the patient to be viewed. The Patient Data sheet (Figure 14-32) will be displayed. 5. Select the Report tab at the bottom of the workbook. The report for the patient will be displayed.
- Page 268 CAPINTEC, INC. CAPTUS ® 4000e Note: If you try to type in any cell in the Formulas or Report sheet, the message in Figure 14-39 will appear. The sheet is protected and can only be changed when the workbook is entered from Setup. Please ignore the second sentence in the message;...
-
Page 269: Cleaning And Maintenance
It is recommended that periodic (every three to five years) re-calibration of the CAPTUS ® 4000e be performed only by Capintec’s Authorized Service Center to guarantee the instrument’s high reliability is maintained. Contact Capintec’s only Authorized Service Center (800) ASK-4CRC... -
Page 270: Cleaning And Disinfecting
CAPINTEC, INC. CAPTUS ® 4000e CLEANING AND DISINFECTING CAUTION: • DISCONNECT THE POWER BEFORE CLEANING. • TO AVOID ELECTRICAL SHOCK OR DAMAGING OF THE CAPTUS ® 4000e, NEVER ALLOW WATER OR LIQUIDS TO PENETRATE THE DETECTOR HOUSINGS OR THE COMPUTER SYSTEM ENCLOSURES. - Page 271 CAPINTEC, INC. CAPTUS ® 4000e COMPUTER CASE – Use a soft cloth lightly dampened with water, window cleaner • or mild detergent. Wipe the surfaces of the computer case and the keyboard keys as necessary to remove dust, fingerprints, and/or smudges.
-
Page 272: Disinfecting Instructions
Detectors when handled carefully. If the unit fails to pass the Auto Calibration or any other test, the user should not attempt to perform any adjustments to the system. In such an event, please contact Capintec’s only Authorized Service Center for further assistance. -
Page 273: Disposal
4000e DISPOSAL The following items should be taken into consideration before disposing. These items should be disposed of in accordance with local and national regulations. Please contact Capintec, Inc. or an authorized disposal company to decommission your equipment. Figure 15-1... -
Page 274: Servicing
Other than the printer’s ink cartridges and the Isolation Transformer’s fuses (reference FUSE SERVICING on page 15-9), there are no user serviceable parts contained in the system. Every five years, the system should be returned to Capintec’s only Authorized Service Center for a complete verification. -
Page 275: Spring-Arm Inspection
Cable (4) damage, d. Loose Snap Ring (2), and e. Loose Pin Retention Plate (5). If any of these conditions exist, stop use immediately and contact Capintec’s only Authorized Service Center for information on replacement items, instructions or on-site service. Figure 15-3... - Page 276 Spring-Arm. The measurement should be between 36 and 37 inches (91–94 cm). If the Spring-Arm does not move smoothly or if the travel measurements cannot be achieved, contact Capintec’s only Authorized Service Center for information on replacement items, instructions or on-site service. Figure 15-4...
-
Page 277: Computer Battery Replacement
CAPINTEC, INC. CAPTUS ® 4000e Computer Battery Replacement To replace the battery located inside the computer, please contact the computer manufacturer for proper procedure and battery type. FUSE SERVICING CAUTION: FOR CONTINUED PROTECTION, REPLACE ONLY WITH SAME TYPE AND RATING OF FUSE(S). A FIRE HAZARD MAY EXIST IF THE WRONG RATING OF FUSE IS INSTALLED. - Page 278 CAPINTEC, INC. CAPTUS ® 4000e 4. Once the door is opened, again use a screwdriver to pry out the fuse drawer behind the door. The drawer will slide out. (Refer to Figure 15-6) Insert screwdriver here and pry fuse drawer out...
-
Page 279: Computer And Printer Fuses
CAPINTEC, INC. CAPTUS ® 4000e 6. Remove the blown fuse(s) from the holder and replace it/them with the fuses specified on the Isolation Transformer label. When installing the new fuse(s), verify that they are contacting the correct terminals as shown in Figure 15-8. -
Page 280: Accessories And Replacement Parts
CAPINTEC, INC. CAPTUS ® 4000e ACCESSORIES AND REPLACEMENT PARTS The following accessories and replacement parts are available from Capintec. Call (800) ASK-4CRC Capintec’s only Authorized Service Center at for answers to your questions or to place an order. • Ream of Paper – 500 sheets ............9282-0011 •... -
Page 281: Shipping
SHIPPING If for any reason the CAPTUS ® 4000e must be returned to Capintec, the shipping carton must contain the following or equivalent labeling as shown in Figure 15-9. Label stipulating the maximum environmental conditions for safe storage and shipment. -
Page 282: Cap-Dicom
License Information The DICOM software module requires a license to function. If you have purchased DICOM with the system, Capintec will have provided the correct license to input into the Module Licenses Screen. From the CAPTUS® 4000e Main screen, select the Setup button. Figure 16-1 Setup Window will appear. - Page 283 CAPINTEC, INC. CAPTUS ® 4000e Figure 16-1 Setup Window Select the Module Licenses button. Figure 16-2 Module Licenses window will appear. Figure 16-2 Module Licenses window 16-2 DICOM August 20...
- Page 284 CAPINTEC, INC. CAPTUS ® 4000e The license key must be entered in one the boxes in the Figure 16-2 Module Licenses window. If a valid license key is entered, “CapDICOM” is displayed next to the license key box. If the license key entered is invalid “Invalid License” is displayed next to the license key box.
-
Page 285: Settings
CAPINTEC, INC. CAPTUS ® 4000e Figure 16-4 DICOM Setup Window Settings On Figure 16-4 DICOM Setup Window, select the Settings button. Figure 16-5 DICOM Settings Window will appear. 16-4 DICOM August 20... -
Page 286: Ae Title
CAPINTEC, INC. CAPTUS ® 4000e Figure 16-5 DICOM Settings Window AE Title AE Title is a common abbreviation for the DICOM term Application Entity. The DICOM Application Entity identifies this software application on a DICOM network. The Application Entity is usually assigned by the network administrator. -
Page 287: Study Date
CAPINTEC, INC. CAPTUS ® 4000e Study Date The Study Date allows the user to set different methods for the study date to be collected from the Captus or Worklist information. Institution Information This allows the user to enter information about their institution and department. The information is placed in the DICOM header. - Page 288 CAPINTEC, INC. CAPTUS ® 4000e Figure 16-6 This Worklist Provider window is used to specify the DICOM Modality Worklist Provider configuration information. The system supports more than one provider. Input the information provided on the Site Survey or as directed by your PAC’s administrator.
- Page 289 CAPINTEC, INC. CAPTUS ® 4000e Service List The service list is automatically filled in. It should only be changed by support personnel. Worklist Threshold Sets the max number of DICOM Worklist records that can be retrieved before the Cap-DICOM software issues a Query Cancel request to the DICOM Worklist provider.
-
Page 290: Editing Worklist Provider
CAPINTEC, INC. CAPTUS ® 4000e Figure 16-7 Editing Worklist Provider To select a Worklist Provider to be edited, use the and arrow keys or the Page Up and Page Down keys to move up or down in the Worklist Provider list. If the list is long, the scroll bar is also available. -
Page 291: Deleting Worklist Provider
CAPINTEC, INC. CAPTUS ® 4000e Figure 16-8 Click in the text box to be edited and type over the existing information. After entering the data, select the Echo button to confirm that the WORKLIST SERVER is communicating with the CAPTUS ®... -
Page 292: Storage Provider
CAPINTEC, INC. CAPTUS ® 4000e Storage Provider Adding Storage Provider Now you are ready to add a Storage Provider. On Figure 16-7, select the Add Storage Provider button. Figure 16-9 will appear. Figure 16-9 This Storage Provider window is used to specify the DICOM Modality Storage Provider configuration information Input the data provided to you on the Site Survey or at the directions of your PAC’s... - Page 293 CAPINTEC, INC. CAPTUS ® 4000e Port This indicates the TCP/IP port number of the DICOM Modality Worklist Provider. (e.g., "104") Service List The service list is automatically filled in. It should only be changed by support personnel. Echo Button This button performs a DICOM Echo using the information configured on this page and the local DICOM settings page to test the DICOM association to the Worklist Provider.
-
Page 294: Editing Storage Provider
CAPINTEC, INC. CAPTUS ® 4000e Once saved, the Storage Provider will be displayed in the list as shown in Figure 16-11. Figure 16-11 The system is now setup to retrieve patient information and send completed reports/data to the DICOM SERVER. -
Page 295: Deleting Storage Provider
CAPINTEC, INC. CAPTUS ® 4000e Figure 16-12 Click in the text box to be edited and type over the existing information. After entering the data, select the Echo button to confirm that the DICOM SERVER is communicating with the CAPTUS ®... -
Page 296: Importing Patients
CAPINTEC, INC. CAPTUS ® 4000e IMPORTING PATIENTS The next step is to test the setup and make sure it is pulling patient information and sending the completed data back to the DICOM SERVER. Query Worklist 1. On Figure 16-11, select the Exit button. Figure 16-3 Setup window with DICOM buttonScreen will be displayed. - Page 297 CAPINTEC, INC. CAPTUS ® 4000e Figure 16-14 3. From Figure 16-14, select the DICOM Worklist button. Figure 16-15 DICOM Patient Worklist Screen will appear. Figure 16-15 DICOM Patient Worklist Screen 16-16 DICOM August 20...
-
Page 298: Start Date/End Date
CAPINTEC, INC. CAPTUS ® 4000e 4. Query Worklist Provider provides an interface for the user to perform a query of the Worklist provider on the hospital PACs or RIS system. Select the Query Worklist Provider button. Figure 16-16 will appear. -
Page 299: Accession Number, Patient Id And Patient Name
CAPINTEC, INC. CAPTUS ® 4000e Accession Number, Patient Id and Patient Name These provide the user with the means to search for a specific name, ID or Accession number in the Worklist. DICOM requires that all Worklist Providers to support queries by Patient Name, Patient ID and Accession number but not all providers have this functionality. -
Page 300: Viewing The Dicom Report
CAPINTEC, INC. CAPTUS ® 4000e Figure 16-18 VIEWING THE DICOM REPORT Once the study is completed, the DICOM report can be viewed. Figure 16-19 August 20 DICOM 16-19... - Page 301 CAPINTEC, INC. CAPTUS ® 4000e From Figure 16-19, select the Report button. Figure 16-20 will appear. Figure 16-20 Select the DICOM Report button. The DICOM Report window will appear displaying the information that will be transferred to the DICOM Server. Refer to Figure 16-21 DICOM Report Window –...
-
Page 302: Exporting A Study
CAPINTEC, INC. CAPTUS ® 4000e Select the Next Page button to see the rest of the report. Figure 16-22 DICOM Report Window – Page 2 EXPORTING A STUDY Once the study is completed it is ready to export to the DICOM server. -
Page 303: Verifying Study
CAPINTEC, INC. CAPTUS ® 4000e Select the Exit button to exit the Thyroid Uptake screen. The CAPTUS ® 4000 Main screen will be displayed. VERIFYING STUDY Check the PAC’s Server to confirm that the report was sent over. Figure 16-24... - Page 304 CAPINTEC, INC. CAPTUS ® 4000e INDEX Absorbed Dose ALERT, 9-1 Battery Replacement Computer, 15-9 Adding Patients, 9-11 Counting Parameters Frame, 9-5 Bioassay, 10-1 Counting Time, 9-5 Adding Staff Members, 10-9 Creating an Archive, 9-21 Cross Contamination, 10-9 Data Acquisistion, 9-20...
- Page 305 CAPINTEC, INC. CAPTUS ® 4000e Count Selected Patients, 9-17, 14-12 Print / View History, 5-10, 5-15, 5-19, Counting Setup, 12-7 5-22 Create Archive, 9-21, 13-3 Print All, 10-17 Cs137 Efficiency, 5-5, 5-12 Print All Listed, 13-8 Print Latest Results, 10-15, 10-17...
- Page 306 CAPINTEC, INC. CAPTUS ® 4000e Time Activity Analysis, 11-1 Cross Contamination ratios, 10-8 Unselect All, 8-22 Cursor Readout, 12-2 Update, 4-12 Custom Protocols, 14-1 View, 14-7 Adding, 14-3 View Selection, 13-7 Adding Patients, 14-8 Wipe Test, 8-1 Creating a Workbook, 14-16...
- Page 307 CAPINTEC, INC. CAPTUS ® 4000e Displaying Data by Date, 4-13 Edit, 9-9 Displaying Selected User, 4-12 New, 9-6 Disposal, 15-5 Select, 9-6 Incident / Site ID, 9-4 Dose Administration, 7-4 Inspection Collimator Block, 15-6 Edit Incident, 9-9 Spring-Arm, 15-7 Editing Storage Provider, 16-13...
- Page 308 CAPINTEC, INC. CAPTUS ® 4000e Acquisition, 12-2 Changing Detectors, 12-3 Count Data, 12-2 Operator Profile, 2-2 Counting Setup, 12-7 Customizing Settings, 12-14 Training, 2-2 Export, 12-12 Export Raw Spectrum, 12-13 Gain, 12-15 Parameters Chi-Square Test, 5-4 High Voltage, 12-15 Constancy Test, 5-4...
- Page 309 CAPINTEC, INC. CAPTUS ® 4000e Real Time, 12-7 Adding, 16-11 recalibration, 1-6 Editing, 16-13 Regulatory Listings, 2-10 Study Exporting, 16-21 Repetitions Frame, 9-5 Replacement Parts, 15-12 Verify, 16-22 Report Study Date, 16-6 Log On, 4-11 Summary Reports, 8-24 Printing, 4-13...
- Page 310 CAPINTEC, INC. CAPTUS ® 4000e Operator, 2-2 Editing Location Information, 8-8 Exiting Search, 8-23 Exiting Summary Reports, 8-30 Exporting Results, 8-22 User Delete, 4-12 Isotope Efficiency, 8-30 Displaying Selected, 4-12 Measurements, 8-12 Invalidate, 4-13 Printing Results, 8-23 Revalidate, 4-13 Printing Summary Reports, 8-29...
- Page 311 NEW PRODUCT WARRANTY Conditions and Limitations CAPINTEC warrants our products to be free from defects in material and workmanship for a period of 24 months after delivery to the original buyer (unless indicated otherwise). Our obligation under this warranty is limited...
- Page 312 Phone (800) ASK-4CRC (201) 825-1336 declare under our sole responsibility that the product CAPTUS 4000e THYROID UPTAKE SYSTEM (including use with the Hewlett-Packard Officejet printer) to which this declaration relates is in conformity with the MDD 93/42/EEC as transposed into Swedish national law LVFS 2003:11.
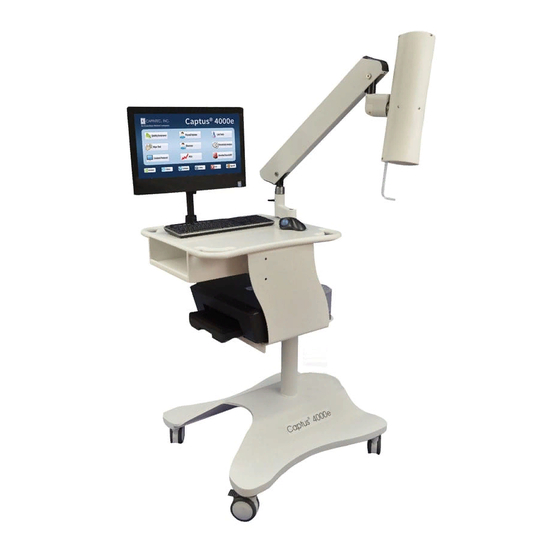


Need help?
Do you have a question about the CAPTUS 4000e and is the answer not in the manual?
Questions and answers