Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for I-Tech I-TECH LA8000
- Page 1 USER MANUAL MNPG179 Rev. 2 of 03/07/2019 Laser therapy...
-
Page 3: Table Of Contents
Summary SUMMARY TECHNICAL INFORMATION NFORMATION ON THE USER MANUAL ANUFACTURER ECLARATION OF CONFORMITY LASSIFICATION URPOSE AND SCOPE ECHNICAL FEATURES EVICE AND COMMAND DESCRIPTION ABELS Packaging content PURPOSE NTRODUCTION TO THE TECHNOLOGY NDICATIONS ONTRAINDICATIONS ARNINGS ATIENT PREPARATION NSTRUCTIONS FOR USE !Warning! Connections Start-up and protection password Main menu... - Page 4 IACER Srl MNPG179...
-
Page 5: Technical Information
It contains general information on the operation, precautionary practices, and maintenance information of the device I-TECH LA8000/10000 The user manual has to be considered as part of the device and it must be stored for future references until its disposal. The user manual must be always kept at hand for quick reference and correctly stored. -
Page 6: Manufacturer
MED24021 from the Notified Body no. 0476, Kiwa Cermet Italia Spa). Declaration of conformity I.A.C.E.R. S.r.l Via S.Pertini 24/A – 30030 Martellago (Ve), Italia herewith declares under its own responsibility, that the products I-TECH LA8000 I-TECH LA10000 UMDNS Code: 12299 Batch no.: Series no.: have been designed and manufactured according to the European Medical Device Directive 93/4/EEC (transposed in Italy by the D.Lgs. -
Page 7: Classification
I-TECH LA8000/10000 is an active therapeutic device, not invasive, used especially by physiotherapists, physicians and pain therapists. The use of I-TECH LA8000/10000 is reserved to those professionals, who can guarantee thanks to their training a proper and completely safe use of the device. -
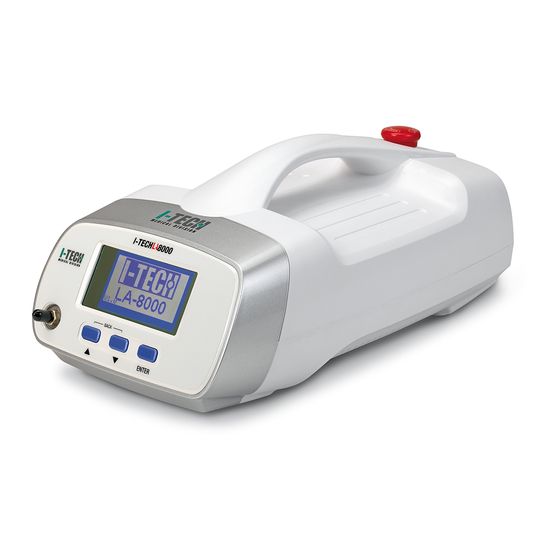
Page 8: Technical Features
In fact, the operator must be properly qualified and must pass an adequate training course or he/she must operate under the guidance of a qualified physician in order to use the device in complete safe condition for the patient under treatment. The device can be used both in hospitals and in clinics, unless the operator is qualified to use such equipment and the conformity to the statements declared in the manual is respected. - Page 9 Characteristic Specification Programmable treatment time Between 1 second and 30min Free memory External dimensions (width x height x depth) 18x18x35cm Weight of the body of the machine Distance between the laser emission point Around 38 mm and the skin Spot area Around 10mm ±3% environmental...
-
Page 10: Device And Command Description
Device and command description IACER Srl MNPG179... -
Page 11: Labels
Labels IACER Srl MNPG179... - Page 12 Label Label Meaning Meaning LABEL 1 LABEL 2 I-TECH Batch n..Date:..Probe LA8000 - LA10000 Placed on one side of the device, Placed on the connector’s tip, it indicates batch number and date. it indicates “Warning, laser beam”. LABEL 4...
- Page 13 Label Label Meaning Meaning LABEL 9 LABEL 10 FUSES T2.5A 230V AC, 50Hz It indicates the position of the It indicates the power supply. safety fuses. LABEL 12 LABEL 11 INTERLOCK It indicates the interlock socket. It indicates the emergency stop button. LABEL 13 It indicates the position of the laser aperture.
-
Page 14: Packaging Content
(device protected against external bodies having a IP20 diameter ≥12,5mm and against the vertical falling of water drops). Obligation to wear protective glasses. Packaging content The I-TECH LA8000/LA10000 box contains: − n.1 user manual, − n.1 power supply cable, shuko plug; −... -
Page 15: Purpose
Introduction to the technology The evolution of light The new I-TECH LA8000/10000 laser with tip permits the direct application of the laser beam to the affected area with the utmost precision. In this way, laser therapy can be carried out to effectively stimulate regeneration in the... - Page 16 4. anti-edematous effect, with stimulation of the lymphatic system; 5. analgesic effect: increase of the threshold of perception of the nerve endings. The I-TECH LA8000/10000 is, therefore, a laser with the following characteristics: a power rating of up to 8000mW, in the case of the LA8000, or...
-
Page 17: Indications
Indications The fields of application of the I-TECH LA8000/10000 laser therapy equipment are: 1. Sports traumatology Strained or pulled muscles, sprained joints, epicondylitis, tendinitis and enthesitis, bruising, hematomas and skin hemorrhages, bursitis. 2. Rheumatic disorders Arthritis, sciatica, scapular-humeral periarthritis, arthropathies of... -
Page 18: Warnings
Treatments over the sympathetic ganglia, the vagus nerve and the heart region in patients with heart disease: laser therapy can affect the neural function to a significant degree and is, therefore, counter indicated in this region of the body in patients with heart disease. Other contraindications: Atopic dermatitis and acute eczema. - Page 19 I-TECH LA8000, making sure not to touch the end of the fiber with your fingers as this could dirty the laser input and prevent the correct flow of the laser beam, causing power losses and overheating between the connection of the tip and the I-TECH LA8000.
-
Page 20: Patient Preparation
Insert the INTERLOCK key in order to run the machine. Do not use accessories other than the original ones provided: non- original ones can damage the machine and render the warranty null and void. If any problems or difficulties arise during installation, please contact the technical assistance service at IACER srl. - Page 21 MOVE THE HANDPIECE TO SCAN THE TREATED AREA. Laser treatments must be carried out under the close supervision of the operator and with the patient fully conscious and able to provide feedback on the effects of the machine. IACER Srl cannot be held responsible for accidents due to a failure to observe this requirement.
-
Page 22: Connections
Turning the device on powers the I-TECH LA8000/10000 and the backlit LCD display lights up, indicating that the equipment is ready to start. -
Page 23: Main Menu
PIN the display will show the main menu. Main menu The I-TECH LA8000/10000 laser therapy equipment can be used in either of two work modes: with emission of the beam in POINT mode or in SCANNING mode. -
Page 24: Program Selection
The "Free" section on the I-TECH LA8000/10000 offers the possibility of customizing 10 protocols - 5 for "Trigger Point" mode and 5 for "Scanning" mode; all changes can be saved in the internal memory. This means that the operator can find his/her favorite settings even after a long period of downtime. - Page 25 6. It is possible to end the session at any time during treatment by releasing the actuator (to pause the equipment) and pressing the ENTER key; the I-TECH LA8000/10000 automatically disables the laser and returns to the main menu. PROGRAMS’ LIST...
-
Page 26: Selection Of Free Programs
18J/cm Selection of free programs I-TECH LA8000/10000 enables the user to set manually the parameters of the protocols in FREE mode. In fact, the FREE section offers ten customizable spaces for changing the work parameters. The first 5 spaces are for treating patients in conventional "Trigger Point"... - Page 27 UP and DOWN keys or just confirm them by pressing the ENTER key. To go back both in the main menu and for the parameters press the BACK key. The various parameters of the I-TECH LA8000/10000 are explained in the table below:...
-
Page 28: End Of Treatment
After setting all the parameters, press ENTER to tell the I-TECH LA8000/10000 that all the parameters have been entered and the software prompts the operator to press ENTER to start therapy. The I-TECH LA8000/10000 then runs as indicated from step 4 in the previous section. -
Page 29: Device's Care
Use the UP and DOWN keys to select the first number of the PIN and then press ENTER to confirm. Repeat the same operation to change the second, third and fourth number. − At last press ENTER key to confirm the new password. •... - Page 30 After cleaning the external parts of the box, dry them with care before restarting the equipment. Do not, for any reason, disassemble the equipment for the purpose of cleaning or inspection: there is no need to clean inside the I-TECH LA8000 IACER Srl MNPG179...
- Page 31 machines and, in any case, the machines should be opened only by authorized, specially trained technical staff from IACER Srl. HANDEL CLEANING The optical fiber laser handpiece is a delicate component which requires suitable daily maintenance. The following recommendations should be observed to protect the fiber and lens from damage.
-
Page 32: General Indications For A Proper Use
General indications for a proper use After use, the operator must always turn off and unplug the equipment and check the condition of the optical fiber and the lens of the laser output on the handpiece-applicator. In the event of any damage or impurities, it is recommended to set the device aside and contact the manufacturer. -
Page 33: Troubleshooting
Troubleshooting The I-TECH LA8000/10000 laser therapy machines were designed and created using state-of-the-art technology and quality components to ensure efficient and reliable performances. Should any operational issue occur, however, the operator is recommended to consult the guide below before contacting an authorized assistance center. -
Page 34: Disposal
Please contact IACER Srl or its authorized service centers for repairs and further information. !CAUTION! DO NOT open the device: the HIGH VOLTAGE inside can be DANGEROUS. Disposal The therapeutic laser devices I-TECH LA8000/LA10000 were designed and engineered to have minimal negative environmental impact, in IACER Srl MNPG179... -
Page 35: Warranty
consideration of their performance and safety requirements, following the disposition given by the European Directive 2012/19/EU, regarding the waste electrical and electronic equipment. Rigorous standards were followed in order to minimize the amount of waste, use of toxic materials, noise, non-required radiation and energy consumption. -
Page 36: Support
use of non-original accessories. The warranty is supplied ex works. Should you need to return the goods then please note the packing instructions as follows. Enclose a copy of the purchasing receipt. Before sending the machine back for suspected malfunction, we recommend that first you carefully consult sections regarding MAINTENANCE and TROUBLESHOOTING of the manual, as a large part of the problems and faults are usually due to inadequate maintenance or small technical problems... -
Page 37: Electromagnetic Interferences And Electromagnetic Compatibility Tables
Electromagnetic interferences electromagnetic compatibility tables The I-TECH LA8000/LA10000 equipment has been designed and manufactured according to the ELECTROMAGNETIC COMPATIBILITY DIRECTIVE 2014/30/EU with the aim of providing adequate protection from harmful interference when installed in homes and health establishments. The equipment does not generate significant radio frequency energy and is adequately immune to radiated electromagnetic fields. - Page 38 COMPATIBILITY ELECTROMAGNETIC TABLES Guidance and manufacturer’s declaration – ELECTROMAGNETIC EMISSIONS The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Electromagnetic environment –...
- Page 39 Guidance and manufacturer’s declaration – ELECTROMAGNETIC IMMUNITY The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Electromagnetic IEC 60601-1-2 Compliance Immunity test environment –...
- Page 40 Guidance and manufacturer’s declaration – ELECTROMAGNETIC IMMUNITY The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. Electromagnetic IEC 60601-1-2 Compliance Immunity test environment –...
- Page 41 Guidance and manufacturer’s declaration – ELECTROMAGNETIC IMMUNITY The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment. IEC 60601-1-2 Compliance Electromagnetic Immunity test Test level...
- Page 42 I-TECH LA8000 should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the I-TECH LA8000. Over the frequency range 150kHz to 80MHz, field strengths should be less than [V ] V/m.
- Page 43 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. I-TECH LA8000/LA10000. All rights reserved I-TECH LA8000/LA10000 and the logo are property exclusively of I.A.C.E.R. Srl and registered.
















Need help?
Do you have a question about the I-TECH LA8000 and is the answer not in the manual?
Questions and answers