Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Tactile Medical Actitouch
- Page 1 A C T I TO U C H S Y S T E M ® U S E R G U I D E Adaptive Compression Therapy...
-
Page 2: Table Of Contents
System ..... . 9 9.3 Electromagnetic 5.5 Switching On the ACTitouch Interference ....28 System in Sustained Compression Mode . -
Page 3: Component List
CHAPTER 1 Component List Before first use, ensure that the following ACTitouch® System components are accessible: Compression Sleeve Power Adapter/Charger Control Unit Undersocks (Three [3] socks included) User Guide (Not shown) Customer Service Toll-Free: 866.435.3948... -
Page 4: Product Description
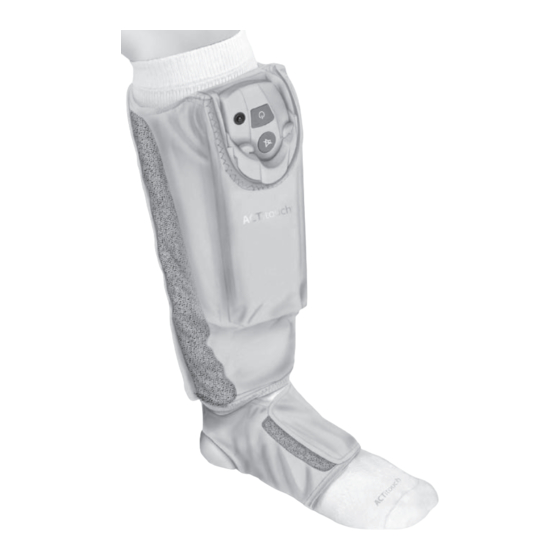
CHAPTER 2 Product Description The ACTitouch System applies pneumatic compression to the lower leg, ankle, and foot. It consists of four (4) main parts: 1. The Compression Sleeve consists of four (4) chambers that inflate with air to apply pressure to the leg. Its simple wrap-around design with hook and loop fasteners means the Compression Sleeve can be fitted to many differently shaped legs and can be applied and removed with ease. -
Page 5: Compression Mode
when the correct pressures are achieved and will hold these pressures until the device is turned off. Every half hour, the pressures are automatically checked and readjusted, if necessary. The patient may hear the pump running for a few seconds Pressure Changes During while the pressure is being checked. -
Page 6: Warnings And Cautions
• Electrical equipment may be hazardous if misused. Do not open or take apart the ACTitouch control unit for any reason or the warranty will be voided. There are no customer serviceable parts in this device. •... -
Page 7: Cautions
Ensure that the device is clean and dry prior to storage. • Do not immerse the ACTitouch System in water, or spill liquid on the control unit. The device is not waterproof, and exposure to liquid may damage the control unit or Compression Sleeve. If the device becomes soaked with fluid, discontinue use of the device. -
Page 8: Contraindications
CHAPTER 5 Directions for Use • The ACTitouch System should be worn as recommended and prescribed by the physician. The usual prescribed duration of use will range from 10 to 14 hours per day, including both Sustained Compression and Intermittent Pneumatic Compression Modes. -
Page 9: Functional Controls
Functional Controls ACTitouch Control Unit Status indicator Charging port ON/OFF button MUTE or PAUSE button Liquid crystal display (LCD) ACTitouch Compression Sleeve (Outer Face Up) Control unit release buttons Control unit release handle Control unit housing Loop fasteners Leg section Foot section Customer Service Toll-Free: 866.435.3948... -
Page 10: First Use
5 .3 Charging the Device Before each daily use, the ACTitouch System should be fully charged. It is recommended that the device be charged every night for a minimum of four (4) hours. The control unit should remain inserted in the Compression Sleeve while charging. -
Page 11: Applying The Actitouch
5 .4 Applying the ACTitouch System CAUTION: The ACTitouch Undersock should not be placed in direct contact with an open wound . It is recommended that this device be used in conjunction with an appropriate wound dressing before the device is applied (See Chapter 6 Wound Dressings) . - Page 12 5 .4 .2 Inserting the Control Unit into the Compression Sleeve Slide the control unit into its housing on the Compression Sleeve until a click is felt and heard; the control unit is then secured correctly within the sleeve. Correct placement Verify the control unit is correctly assembled by checking to see if the blue markings are visible on the front of the control unit.
- Page 13 The correctly applied foot section will appear as shown. Once the foot section is fastened, lift the leg section of the Compression Sleeve and drape over the leg with the control unit and “ACTitouch” logo positioned over the shin. Wrap the loop fasteners around the back of the calf.
-
Page 14: Switching On The Actitouch System In Sustained Compression Mode
IMPORTANT: If footwear is to be worn during the treatment session, it is advisable for the wearer to put on footwear prior to switching on the ACTitouch System . Do not switch on the device in Sustained Compression Mode unless it is applied to the leg . -
Page 15: Muted Operation In Sustained Compression Mode
• The Compression Sleeve will now inflate. It may take up to five (5) minutes to completely inflate the Compression Sleeve. Remain seated during inflation. To ensure a better fit, pull up and support the Compression Sleeve in place while the chambers inflate. - Page 16 It is recommended that the Intermittent Pneumatic Compression Mode be used for at least two (2) hours per day. When using in Intermittent Pneumatic Compression Mode, the user should be either seated or lying down with the foot elevated and ankle relaxed; not standing. This can be for: •...
-
Page 17: Switching Off The Actitouch
. This is a safety feature of the device, which may be restarted in the normal way (See Section 5 .5 Switching On the ACTitouch System in Sustained Compression Mode) . If the device switches off repeatedly, refer to Chapter 8 Troubleshooting Guide . -
Page 18: Removing The Actitouch
Remove the ACTitouch System prior to showering. If you do not wish to change the dressing when showering, cover the dressing with a waterproof outer layer. -
Page 19: Cleaning, Care And Maintenance
Cleaning the ACTitouch Undersock IMPORTANT: The ACTitouch Undersock is intended for single patient use only . It is recommended that the ACTitouch Undersock be replaced after a maximum of 60 washes . To purchase additional socks please contact Customer Service at 866 .435 .3948 . - Page 20 Do not dry clean DisCide® ULTRA Spray Disinfectant has been demonstrated to effectively disinfect the ACTitouch System. Use DisCide ULTRA Spray or similar disinfectant compliant with OSHA’s Bloodborne Pathogen Standard (29 CFR 1910.1030) and/ or registered with EPA. To disinfect the ACTitouch Compression Sleeve between...
-
Page 21: Storage And Handling
The ACTitouch System is designed to be maintenance free; routine service is not required. CAUTION: Do not open or take apart the ACTitouch control unit for any reason . There are no customer serviceable parts in the ACTitouch System . -
Page 22: Troubleshooting Guide
CHAPTER 8 Troubleshooting Guide The following table provides a troubleshooting guide for the ACTitouch System in the unlikely event of a malfunction. Please refer to Section 8.2 Obtaining Replacement Products and Service for additional information. Problem Possible Cause Corrective Action The Compression 1. - Page 23 Mode . charged. 3. Switch the control unit on. Refer to 3. The control unit has not been Section 5.5 Switching On the ACTitouch switched on. System in Sustained Compression Mode. 4. The MUTE button has been 4. Deactivate muted operation. Refer to activated.
- Page 24 Problem Possible Cause Corrective Action The control unit 1. The control unit has not been 1. Refer to Section 5.4.2 Inserting the Control will not operate securely inserted into the Unit into the Compression Sleeve. in Intermittent Compression Sleeve. 2. Ensure the Power Adapter/Charger is Pneumatic 2.
-
Page 25: Home Use
(1) year from the date of purchase. All other accessories and supplies related to the use of the ACTitouch System are warranted to be free from defects in material and workmanship for their first use. Tactile Medical’s sole obligation in the event of a breach of this warranty is expressly limited to the replacement of defective parts that cannot, in the sole discretion of Tactile Medical, be repaired. -
Page 26: Obtaining Replacement
OBLIGATIONS OR LIABILITIES ON THE PART OF TACTILE MEDICAL FOR DAMAGES ARISING OUT OF OR IN CONNECTION WITH THE USE, REPAIR OR PERFORMANCE OF THE ACTITOUCH SYSTEM. IN NO EVENT SHALL TACTILE MEDICAL BE LIABLE FOR ANY SPECIAL, DIRECT, INDIRECT OR CONSEQUENTIAL DAMAGES. Some states, provinces or countries do not allow exclusion or limitation of incidental or consequential damages, so the above limitation or exclusion may not apply. -
Page 27: Technical Information
CHAPTER 9 Technical Information Equipment Classifications and Standards The ACTitouch System is tested to/complies with the following equipment classifications and standards: U.S. Medical Equipment Classification Class II Degree of Protection Against Electric Shock Class II Classification According to Directive 93/42/EEC Safety UL60601-1 and CAN/CSA C22.2 No.601.1–M90... -
Page 28: Symbols
— generally 20 inches (50 cm) to 40 inches (100 cm) with an illumination of 500 lx minimum. • Call Tactile Medical Customer Service if issues reading the label remain. • Device label not to scale. •... - Page 29 Customer Service Toll-Free: 866.435.3948...
-
Page 30: Interference
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions The ACTitouch System is intended for use in the electromagnetic environment specified below. The customer or the user of the ACTitouch System should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment —... -
Page 31: Electromagnetic
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The ACTitouch System is intended for use in the electromagnetic environment specified below. The customer or the user of the ACTitouch System should assure that it is used in such an environment. Immunity test... - Page 32 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The ACTitouch System is intended for use in the electromagnetic environment specified below. The customer or the user of the ACTitouch System should assure that it is used in such an environment. Immunity...
- Page 33 The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the ACTitouch System can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ACTitouch System as recommended below, according to the maximum output power of the communications equipment.
- Page 36 Hours: Monday through Friday, 7 a.m. – 7 p.m. CT F: 612.355.5101 www.tactilemedical.com ACTitouch and the ACTitouch logo are trademarks of Tactile Systems Technology Inc., DBA Tactile Medical DisCide® is a registered trademark of Palmero Health Care Corporation. ©2017 Tactile Systems Technology Inc., DBA Tactile Medical. All rights reserved.







Need help?
Do you have a question about the Actitouch and is the answer not in the manual?
Questions and answers