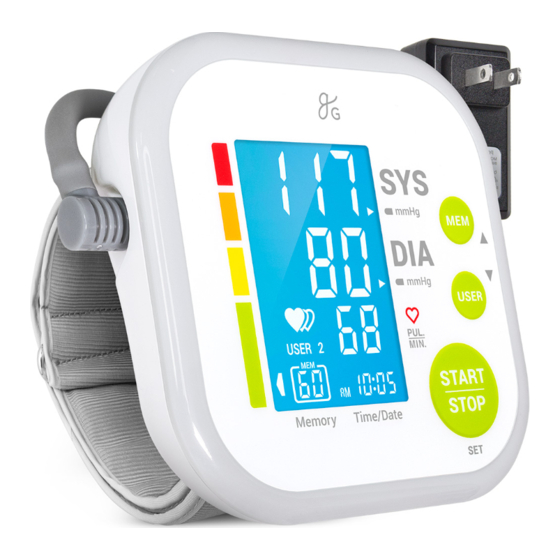
Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for Greater Goods Balance 0600
- Page 2 Symbol for “TYPE BF APPLIED PARTS” Symbol for “THE OPERATION GUIDE MUST BE READ” Symbol for “MANUFACTURER” Symbol for “SERIAL NUMBER” Symbol for “DIRECT CURRENT” Symbol for “MANUFACTURE DATE”...
- Page 3 CAUTION...
- Page 6 CAUTION Remove batteries if the device is not likely to be used for some time. The old battery is harmful to the environment. Do not dispose with other daily trash. Remove the old battery from the device and follow your local recycling guidelines. Do not dispose of batteries in fire.
- Page 9 CAUTION 1. When using this device, pay attention to the following situation, which may interrupt blood flow and influence blood circulation of the patient, thus causing injury to the patient: too frequent and consecutive measurements; the application of the cuff and its pressurization on any arm where intravascular access or therapy, or an arterio-venous (A-V) shunt, is present;...
- Page 13 Systolic blood discharging artery press Diastolic blood entering vein relax...
- Page 15 CAUTION The appearance of the IHB icon indicates that a pulse irregularity consistent with an irregular heart- beat was detected during measurement. Usually this is NOT a cause for concern. However, if the symbol appears often, we recommend you seek medical advice. Please note that the device does not replace a cardiac examination, but serves to detect pulse irregularities at an early stage.
- Page 17 Battery powered mode: Power supply Display mode Oscillographic testing mode Measurement mode Rated cuff pressure: Measurement range (40mmHg-230mmHg) pulse value: (40-199) beat/minute Accuracy Normal working condition Atmospheric pressure: 86kPa to 106kPa Storage & transportation condition Measurement perimeter About 22cm~42cm of the upper arm Approx.175 g(Excluding the dry cells) Net Weight External dimensions...
- Page 18 Risk management ISO/EN 14971:2012 Medical devices — Application of risk management to medical devices ISO/EN 15223-1:2012 Medical devices. Symbols to be Labeling used with medical device labels, labelling and information to be supplied. General requirements EN 1041: 2008 Medical equipment manufacturers to User manual provide information IEC 60601-1: 2005+A1:2012 Medical electrical...



Need help?
Do you have a question about the Balance 0600 and is the answer not in the manual?
Questions and answers
My BP cuff starts on its own and I can’t stop it. If I unplug it and replug it in again it starts inflating and won’t turn of
If your Greater Goods Balance 0600 BP cuff starts inflating on its own and won't turn off, you should remove the batteries immediately to stop the device. This will prevent further inflation and potential injury.
This answer is automatically generated
Hi. I have BP meter/0602 and bp readings are not good with medical readings.
Hi, How good is your bp meter and what is it's.
Error code OUT ?? Why ? Not in manual.