Summary of Contents for Greater Goods Balance 0605
- Page 1 WRIST BLOOD PRESSURE MONITOR MANUAL VIDEO TUTORIAL: greatergoods.com/0605 Email: info@greatergoods.com Phone: (866) 991-8494 Model 0605...
-
Page 2: Table Of Contents
TABLE OF CONTENTS MONITOR COMPONENTS / INSTALLING BATTERIES p. 3-6 SETTING TIME, DATE, AND MEASUREMENT UNIT p. 7-8 TAKING MEASUREMENTS p. 9-10 MEMORY / MANAGING RECORDS p. 11-12 TIPS / MAINTENANCE p. 13-14 ABOUT BLOOD PRESSURE p. 15-16 IRREGULAR HEARTBEAT DETECTOR / BATTERY CAUTIONS p. 17-18 TROUBLESHOOTING p. - Page 3 OUR PROMISE We’re committed to creating 5-star products. If we haven’t delivered on our promise, please contact us. For the best possible experience with your product, please visit greatergoods.com/0605. WARNING: No modification of this equipment is allowed.
-
Page 4: Monitor Components / Installing Batteries
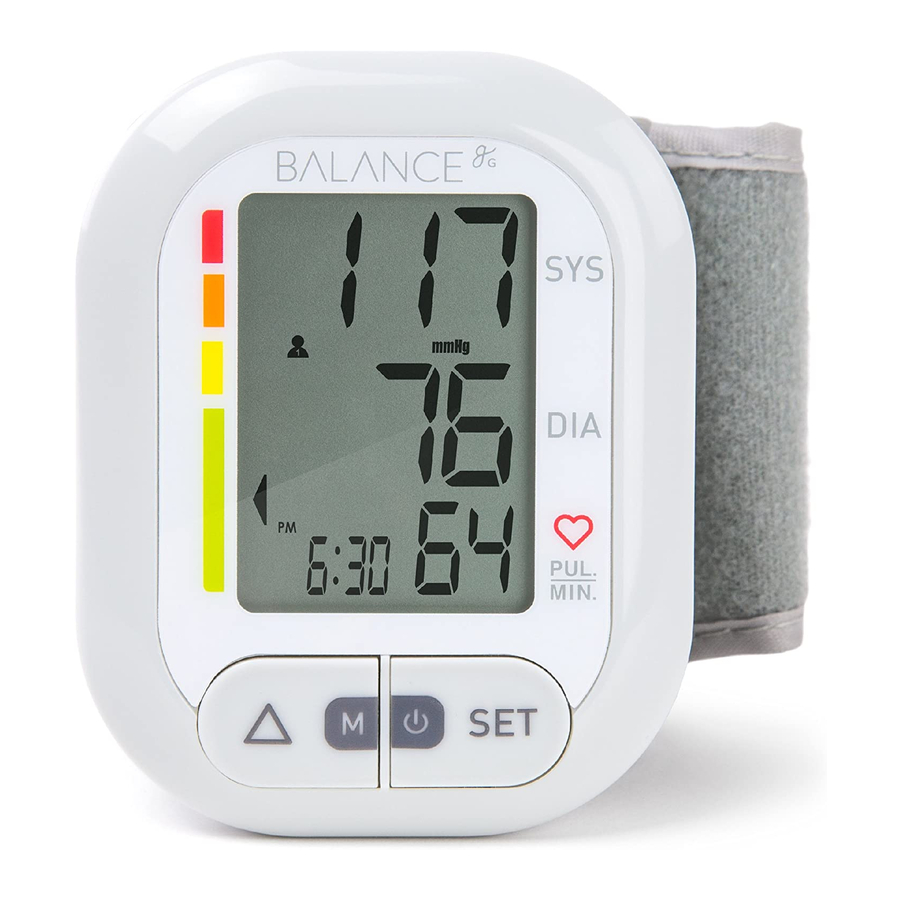
MONITOR COMPONENTS LCD DISPLAY SYSTOLIC GRADE DIASTOLIC TIME PULSE RATE SET / POWER ARROW / MEMORY BUTTON BUTTON NOTE: While the monitor only has two buttons, each button has two functions. Throughout the manual, you will see the buttons referred to by the function being described in that step (e.g. - Page 5 Systolic blood pressure High pressure result Diastolic blood pressure Low pressure result Pulse per minute Beats per minute (BPM) Shaking alert Shaking will result in inaccurate measurements kPa (kiloPascal) Measurement unit of blood pressure mmHg Measurement unit of blood pressure (millimeters of mercury) Irregular Heartbeat Irregular heartbeat...
- Page 6 MONITOR COMPONENTS (cont.) Package Contents: 1. Wrist Blood Pressure Monitor 2. 2×AAA batteries 3. User manual BATTERY CUFF COMPARTMENT (Type BF applied part)
- Page 7 INSTALLING AND REPLACING THE BATTERIES • Slide off the battery cover. • Install the batteries by matching the correct polarity, as shown here. Always use the correct battery type (2 x AAA batteries). • Replace the cover. Replace the batteries whenever the following happen: •...
-
Page 8: Setting Time, Date, And Measurement Unit
SETTING TIME, DATE, AND MEASUREMENT UNIT It is important to set the clock before using your blood pressure monitor, so that a time stamp can be assigned to each record that is stored in the memory. (year: 2014 - 2054 , time format: 24 H /12 H) 1. - Page 9 3. Use the “ARROW” and “SET” buttons like you did in the previous step to confirm MONTH, DAY, TIME FORMAT [24 hours or 12 hours], HOURS, MINUTES, and the MEASUREMENT UNIT. IMPORTANT! Most doctors in the United States use mmHg (millimeters of mercury) as the measurement unit.
-
Page 10: Taking Measurements
FASTEN THE CUFF 1. Remove all accessories (watch, bracelet, etc.) from your wrist. If your physician has diagnosed you with poor circulation in one wrist, use the other one. 2. Roll or push up your sleeve to expose the skin. 3. - Page 11 START THE MEASUREMENT 1. When the blood pressure monitor is off, press the “POWER” button to turn it on. The LCD will display the user ID first. Press the “ARROW” button to change the user ID between User 1 and User 2. Press the “SET” button to confirm, and it will finish the whole measurement for the selected user.
-
Page 12: Memory / Managing Records
NOTE: For a meaningful comparison, try to measure under similar conditions. For example, take daily measurements at approximately the same time, on the same wrist, or as directed by a physician. RECALLING RECORDS 1. When the monitor is off, press the “MEMORY” button to show the user selection screen. - Page 13 DELETE THE RECORDS You can delete all results of the selected user by following the steps below: When the monitor is off, press the “MEMORY” button to show the user selection screen. Use the “ARROW” button to switch between users. Use the “SET”...
-
Page 14: Tips / Maintenance
MAINTENANCE • Store monitor in a dry place. • Avoid contact with water; clean it with a dry cloth if needed. • Do not attempt to clean the cuff with water. • Never submerge monitor or cuff in water. TIPS FOR MEASUREMENT It can cause inaccuracy if the measurement is taken under the following circumstances: •... - Page 15 STANDARD CLASSIFICATION...
-
Page 16: About Blood Pressure
ABOUT BLOOD PRESSURE Q: What is the difference between systolic pressure and diastolic pressure? A: When ventricles contract and pump blood out of the heart, the blood pressure reaches its maximum value in the cycle, which is called systolic pressure. When the ventricles relax, the blood pressure reaches its minimum value in the cycle, which is called diastolic pressure. - Page 17 ABOUT BLOOD PRESSURE (cont.) Q: Why does my blood pressure fluctuate throughout the day? A: Individual blood pressure varies throughout the day. It is also affected by the way you fasten your cuff and your measurement position. Please make sure you measure your blood pressure under the same conditions each time you take a measurement.
-
Page 18: Irregular Heartbeat Detector / Battery Cautions
IRREGULAR HEARTBEAT DETECTOR This Blood Pressure Monitor is equipped with an intelligent function of Irregular Heartbeat Detector (IHB). During each measurement, this equipment records the heartbeat intervals and works out the standard deviation. If the calculated value is larger than or equal to 15, this equipment will light up the IHB symbol on the screen when displaying the results. - Page 19 BATTERY CAUTIONS Remove batteries if the device is not likely to be used for some time. The old batteries are harmful to the environment; do not dispose with other daily trash. Remove the old batteries from the device and follow your local recycling guidelines.
-
Page 20: Troubleshooting
TROUBLESHOOTING Batteries are exhausted Replace with new batteries Display will not light up. Insert the batteries Batteries are inserted correctly incorrectly Display is dim or Batteries are low Replace with new batteries icon is showing Refasten the cuff and then E1 shows on screen The inflation is too low measure again... - Page 21 The pressure of the cuff Relax for a moment and then E5 shows on screen is excessive measure again The voltage of the Measure again. If E6 appears E6 shows on screen batteries is too low again, replace the batteries during inflation The monitor detected E10 or E11 shows...
-
Page 22: Device Specifications
DEVICE SPECIFICATIONS Power supply: 2 AAA batteries (3V DC) Display mode: Digital LCD V.A. 32mm × 45mm Measurement mode: Oscillographic testing mode Measurement range: Rated cuff pressure: 0mmHg~300mmHg(0kPa ~ 40kPa) Measurement pressure: SYS: 60mmHg~230mmHg (8.0kPa~30.7kPa) DIA: 40mmHg~130mmHg (5.3kPa~17.3kPa) Pulse value: (40 -199) beat / minute Accuracy: Pressure: 5˚C - 40˚C within ±... - Page 23 Storage & transportation condition: Temperature: -20˚C to 60˚C Relative humidity: 10% RH to 93% RH Atmospheric pressure: 50 kPa to 106 kPa Measurement perimeter of the wrist: About 5.3 in - 8.4 in Net Weight: Approx. 105 g (Excluding the dry cells) External dimensions: Approx.
-
Page 24: Complied Standards List, Fcc Statement, Emc Guidance
COMPLIED STANDARDS LIST Risk management: ISO/EN 14971:2012 Medical devices — Application of risk management to medical devices Labeling: ISO/EN 15223-1:2012 Medical devices. Symbols to be used with medical device labels, labeling, and information to be supplied. General requirements User manual: EN 1041: 2008 Medical equipment manufacturers to provide information General Requirements for Safety: IEC 60601-1: 2005+A1:2012 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance. - Page 25 Performance requirements and Clinical investigation: IEC 80601-2-30:2009 Medical electrical equipment- Part 2-30: Particular requirements for the basic safety and essential performance of automated non-invasive sphygmomanometers. ANSI/AAMI SP10:2002/A2: 2008 Manual, electronic, or automated sphygmomanometers Software life-cycle processes: IEC/EN 62304:2006+AC: 2008 Medical device software - Software life cycle processes Usability: IEC 62366 Medical devices - Application of usability engineering to medical devices (IEC 62366:2007) IEC 60601-1-6 Medical electrical equipment -...
-
Page 26: Fcc Statement
FCC STATEMENT This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) this device may not cause harmful interference and (2) this device must accept any interference received, including interference that may cause undesired operation. - Page 27 LABEL INFORMATION Symbol for “MANUFACTURE Symbol for “THE OPERATION DATE” GUIDE MUST BE READ” Symbol for “CAUTION” - These notes Symbol for “TYPE BF APPLIED must be observed for advice PARTS” or to prevent any damage to device. Symbol for “ENVIRONMENT Symbol for “MANUFACTURER”...
-
Page 28: Safety Information / Indications For Use
SAFETY INFORMATION This device is intended for adult use only. This device is intended for non-invasive measuring and monitoring of arterial blood pressure. It is not intended for use on extremities other than the wrist or for functions other than obtaining a blood pressure measurement. Do not confuse self-monitoring with self-diagnosis. - Page 29 If the cuff pressure exceeds 40 kPa (300 mmHg), the unit will automatically deflate. Should the cuff not deflate when pressures exceeds 40 kPa (300 mmHg), detach the cuff from the wrist and press the START/ STOP button to stop inflation. The equipment is not AP/APG equipment and not suitable for use in the presence of a flammable anesthetic mixture with air of with oxygen or nitrous oxide.
- Page 30 SAFETY INFORMATION (cont.) The operator shall not touch output of batteries and the patients simultaneously. Do not use the monitor under the conditions of strong electromagnetic field (e.g. medical RF equipment) that radiates interference signal or electrical fast transient / burst signal. The user must check that the equipment functions safely and see that it is in proper working condition before being used.
- Page 31 Please use the device under the environment which was provided in the user manual. Otherwise, the performance and lifetime of the device will be impacted and reduced. During use, the patient will be in contact with the cuff. This device will not cause any potential allergic reaction or contact injury. The device doesn’t need to be calibrated with two years of reliable service.
- Page 32 SAFETY INFORMATION (cont.) The device has been evaluated clinically using manual cuff/stethoscope auscultation as the reference. Blood pressure measurements determined with this device are equivalent to those obtained by a trained observer using the cuff / stethoscope auscultatory method, within the limits prescribed by the American National Standard, manual, electronic, or automated sphygmomanometers.
- Page 33 INDICATIONS FOR USE The Balance Blood Pressure Monitor is intended for use in measuring blood pressure and heartbeat rate with wrist circumference ranging from 13.5 cm to 21.5 cm ( about 5˝-8½˝ ). It is intended for adult indoor use only. FEATURES: Systolic blood pressure Diastolic blood pressure...
-
Page 34: Warranty
WARRANTY INFORMATION Your Blood Pressure Monitor is warranted by the manufacturer against defects in materials and workmanship for two (2) years from the original purchaser from the date of purchase. Proof of purchase is required. The warranty is void if the product has been subjected to mechanical damage or mistreatment, such as immersion. - Page 35 Manufactured by: Guangdong Transtek Medical Electronics Co., Ltd Company: Guangdong Transtek Medical Electronics Co., Ltd Address: Zone A, No.105, Dongli Road, Torch Development District, Zhongshan, 528437, Guangdong, China...
- Page 36 Distributed by: Greater Goods LLC. (toll free) 866-991-8494 125 N Main St Suite 202 greatergoods.com/0605 St Charles, MO 63301...




Need help?
Do you have a question about the Balance 0605 and is the answer not in the manual?
Questions and answers
How do I sync to my iPhone
how can I download the blood pressure data from the Balance app to a file to send to my doctor?