Table of Contents
Advertisement
Quick Links
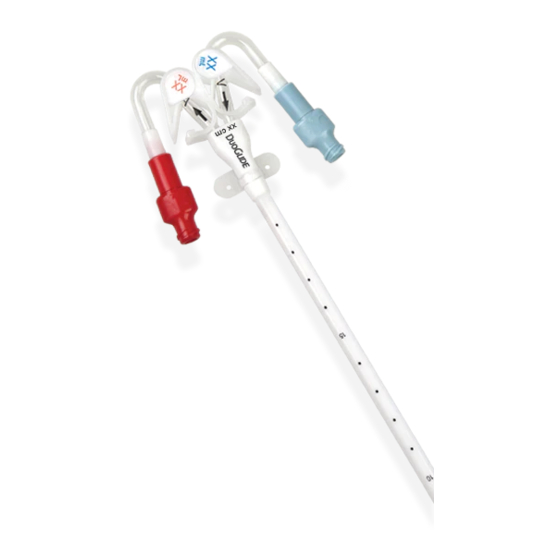
DESCRIPTION
The catheter is divided into two separate round lumens permitting continuous blood flow with one puncture.
All DuoGlide* catheters are made of thermosensitive polyurethane, which softens when exposed to body temperature.
INDICATIONS FOR USE
DuoGlide* Dual Lumen catheters are indicated for use in attaining short term (less than 30 days) vascular access for hemodialysis, hemoperfusion
and apheresis therapy via the jugular, subclavian or femoral vein.
CONTRAINDICATIONS
• The catheter is intended for short-term vascular access only and is not to be used for any purpose other than indicated in these instructions.
ChloraPrep* Solution One-Step Applicator Contraindications
• Do not use in children less than 2 months of age because of the potential for excessive skin irritation and increased drug absorption.
• Do not use on patients with known allergies to chlorhexidine gluconate or isopropyl alcohol.
• Do not use for lumbar puncture or allow contact with meninges.
• Do not use on open skin wounds or as a general skin cleanser.
USA Only
WARNINGS
• SUBCLAVIAN ONLY. Pinch-off Prevention: Percutaneous insertion
of the catheter must be made into the axillary-subclavian vein
at the junction of the outer and mid-third of the clavicle lateral
to the thoracic outlet. The catheter should not be inserted into
the subclavian vein medially, because such placement can lead to
compression of the catheter between the first rib and clavicle and
can lead to damage or fracture and embolization of the catheter.
Fluoroscopic or radiographic confirmation of catheter tip
placement should be helpful in demonstrating that the catheter is
not being pinched by the first rib and clavicle.
Signs of Pinch-off
Clinical:
• Difficulty with blood withdrawal.
• Resistance to infusion of fluids.
• Patient position changes required
for infusion of fluids or blood withdrawal.
Radiologic: (see table)
•
Grade 1 or 2 distortion on chest X-ray.
Pinch-off should be evaluated for degree of
severity prior to explantation. Patients
indicating any degree of catheter distortion
at the clavicle/first rib area should be followed
diligently. There are grades of pinch-off that
should be recognized with appropriate chest
x-ray as follows:
2,3
• The catheter must not be left in the femoral vein longer than three days. To maintain peak performance it is recommended that
subclavian and jugular catheters be replaced after four weeks.
• Alcohol or alcohol-containing antiseptics (such as chlorhexidine) may be used to clean the catheter/skin site; however, care should be
taken to avoid prolonged or excessive contact of the catheter with the solution(s). Solutions should be allowed to completely dry before
applying occlusive dressing.
• Acetone and Polyethylene Glycol (PEG)-containing ointments can cause failure of this device and should not be used with polyurethane
catheters. Chlorhexidine patches or bacitracin zinc ointments (e.g., Polysporin* ointment) are the preferred alternative.
• Do not resterilize the catheter or components by any method. The manufacturer will not be liable for any damages
caused by re-use of the catheter or accessories.
• Place all clamps near the center of the polyurethane extension pieces. Polyurethane may develop cuts or tears if
subjected to excessive pulling or contact with rough edges. Repeated clamping near or on the Luer-lock connectors
may cause tubing fatigue and possible disconnection.
• Intended for Single Use. DO NOT REUSE. Reuse and/or repackaging may create a risk of patient or user infection, compromise
the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure,
and/or lead to injury, illness or death of the patient.
• Repeated over-tightening of bloodlines, syringes and caps will reduce connector life and may lead to connector failure.
• Enzymes in blood and heparin may cause temporary sticking of the extensions when clamped for extended periods
of time. To release, open clamp and slide away, gently rotating the tubing between fingers and thumb until the tubing separates.
• To avoid damage to vessels and viscus, infusion pressures must not exceed 25 psi (172 kPa). The use of a 10 ml or
larger syringe is recommended because smaller syringes generate more pressure than larger syringes.
NOTE: A three pound (13.3 Newton) force on the plunger of a 3 ml syringe generates pressure in excess of 30 psi
(206 kPa) whereas the same three pound (13.3 Newton) force on the plunger of a 10 ml syringe generates less than
15 psi (103 kPa) of pressure.
• Accessories and components used in conjunction with this catheter must incorporate Luer-lock adapters in order to
avoid inadvertent disconnection.
• Catheters should be implanted carefully to avoid any sharp or acute angles which could compromise the opening of the catheter lumens.
Bard Access Systems
Dual Lumen Catheter
Instructions For Use
1
1
Radiologic Signs of Pinch-Off
Grade
Severity
Grade 0
No Distortion
Distortion present
without luminal
Grade 1
narrowing
Distortion present with
Grade 2
luminal narrowing
Grade 3
Catheter transection or
fracture
First Rib
Subclavian Vein
Clavicle
Axillary Vein
Pinch-off Area
Infraclavicular Fossa
Recommended Action
No action.
Chest x-ray should be taken to monitor
progression of pinch-off to grade 2
distortion. Shoulder positioning during
chest x-rays should be noted as it can
contribute to changes in distortion
grades.
Removal of the catheter should be
considered.
Prompt removal of the catheter.
Vertebra
Internal Jugular Vein
Superior Vena Cava
Sternum
Advertisement
Table of Contents

Summary of Contents for Bard DuoGlide
-
Page 1: Instructions For Use
All DuoGlide* catheters are made of thermosensitive polyurethane, which softens when exposed to body temperature. INDICATIONS FOR USE DuoGlide* Dual Lumen catheters are indicated for use in attaining short term (less than 30 days) vascular access for hemodialysis, hemoperfusion and apheresis therapy via the jugular, subclavian or femoral vein. - Page 2 • Before dialysis begins, all connections to the extracorporeal circuit must be checked carefully. During all dialysis procedures frequent visual inspection must be conducted to detect leaks and prevent blood loss or entry of air into the extracorporeal circuit. Excess blood leakage may lead to patient shock.
-
Page 3: Care And Maintenance
8. The flexible end of a guidewire is inserted through the introducer needle into the vein. CAUTION: Do not pull back guidewire over needle bevel as this may sever the end of the guidewire. The introducer needle must be removed first. Also, if unusual resistance is met during manipulation of the guidewire, discontinue the procedure and determine the cause of resistance before proceeding. Withdraw needle and guidewire if cause of resistance cannot be determined. 9. Holding the guidewire securely in place, remove the introducer needle. CAUTION: Do not allow the guidewire to inadvertently advance totally into the vessel. 10. The introducer needle tract is widened by creating a small surgical incision at the skin exit site. The incision should be slightly larger than the wide/flat side of the catheter. 11. Use the Dualator* vessel dilator(s) to dilate the subcutaneous tissues. Dilate 2-3 times with slow, gradual advancements. The larger portion of the Dualator* dilator device must enter the vein prior to catheter insertion. -
Page 4: Troubleshooting
10 Center for Disease Control and Prevention, “Guidelines for the Prevention of Intravascular Catheter-Related Infections, ” Morbidity and Mortality Weekly Report, Aug. 9, 2002, 51(RR-10), 1-32. 11 The Society for Healthcare Epidemiology of America, “Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals, ” Infection Control and Hospital Epidemiology, Oct. 2008, 29(S1): S22-S30. Other references available upon request. An issued or revision date for these intructions is included for the user’ s information. In the event two years have elapsed between this date and the product use, the user can contact Bard Access Systems, Inc. to see if additional product information is available. Revision date: June, 2011. *Bard, Dualator and DuoGlide are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks are the property of their respective owners. © 2011 C. R. Bard, Inc. All rights reserved. Bard Access Systems, Inc.











Need help?
Do you have a question about the DuoGlide and is the answer not in the manual?
Questions and answers