Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Mortara S12
- Page 1 TABLE OF CONTENTS REF 9516-183-50-ENG Rev M S12/S19 SURVEYOR PATIENT MONITORS SERVICE MANUAL Manufactured by Mortara Instrument, Inc., Milwaukee, Wisconsin U.S.A. CAUTION: Federal law restricts this device to sale by or on the order of a physician.
- Page 2 7865 N. 86th Street Milwaukee, Wisconsin 53224 This document contains confidential information that belongs to Mortara Instrument, Inc. No part of this document may be transmitted, reproduced, used, or disclosed outside of the receiving organization without the express written consent of Mortara Instrument, Inc.
-
Page 3: Table Of Contents
TABLE OF CONTENTS GENERAL STATEMENTS ........................... 1 ..........................1 ECHNICAL UPPORT AND ERVICE NOTICES ..............................2 ’ ........................2 ANUFACTURER ESPONSIBILITY ........................2 ESPONSIBILITY OF THE USTOMER ..........................2 QUIPMENT DENTIFICATION ......................2 OPYRIGHT AND RADEMARK OTICES ........................2 THER MPORTANT NFORMATION WARRANTY INFORMATION ........................ - Page 4 LCD R & R S12 ........................ 62 EMOVAL EPLACEMENT LCD R & R S19 ........................ 63 EMOVAL EPLACEMENT (S12 ONLY) ........65 EMOVAL AND EPLACEMENT OF THE PTIONAL HERMAL RITER S19 ...................... 68 PTIONAL HERMAL RITER FOR THE CONFORMANCE TESTING ......................... 79 : .............................
- Page 5 ARDIAC UTPUT MOUNTING ACCESSORIES ......................144 (M-S .............. 144 UICK ISCONNECT ERIES OUNTING OMPONENTS ................144 ALUE ERIES OUNTING OMPONENTS (VHM-25) W ..................145 REMIUM OUNT OMPONENTS S12 R S19) ............ 146 URVEYOR TAND OMPONENTS NOT TO BE USED WITH...
- Page 6 TABLE OF CONTENTS...
-
Page 7: General Statements
1. GENERAL STATEMENTS Technical Support and Service Headquarters Sales Support/ Supplies & Accessories Mortara Instrument, Inc. 7865 North 86th Street Mortara Instrument, Inc. Milwaukee, WI 53224 7865 North 86th U.S.A. Street Milwaukee, WI Tel: 414.354.1600 53224 Tel: 800.231.7437 U.S.A. Fax: 414.354.4760... -
Page 8: Notices
The user of this patient monitor must periodically check the accessories, their functionality and integrity. Equipment Identification Mortara Instrument, Inc. equipment is identified by a serial and reference number on the back of the patient monitor. Care should be taken so that these numbers are not defaced. -
Page 9: Warranty Information
Product/s. If Mortara should be found liable to anyone under any theory (except the expressed warranty set forth herein) for loss, harm, or damage, the liability of Mortara shall be limited to the lesser of the actual loss, harm, or... - Page 10 WARRANTY INFORMATION EXCEPT AS SET FORTH HEREIN WITH RESPECT TO REIMBURSEMENT OF LABOR CHARGES, A PURCHASER’S SOLE EXCLUSIVE REMEDY AGAINST MORTARA FOR CLAIMS RELATING TO THE PRODUCT/S FOR ANY AND ALL LOSSES AND DAMAGES RESULTING FROM ANY CAUSE SHALL BE THE REPAIR OR REPLACEMENT OF DEFECTIVE PRODUCT/S TO THE EXTENT THAT THE DEFECT IS NOTICED A N D M O R T A R A I S NOTIFIED WITHIN THE WARRANTY PERIOD.
-
Page 11: User Safety Information
Before attempting to use this device for clinical applications, the operator must read and understand the contents of the user manual and other accompanying documents. Inadequate knowledge or training could result in increased risk of harm to users, patients and bystanders, or damage to the patient monitor. Contact Mortara Technical Service for additional training options. -
Page 12: Power Warnings
Power Warnings Only use the Mortara-provided external power adapter with the Surveyor. Ensure that the power adapter is connected to a properly grounded power terminal and the electrical installation complies with local safety requirements for the environment where it is used. - Page 13 Do not leave the in conditions over 60 ºC or in a heated car. Do not attempt to crush or drop the device. Only use the approved Mortara battery pack with the Surveyor monitor. Follow the instructions in the disposal section of this manual when the Surveyor monitor is taken out of service.
-
Page 14: Accessories, Cables, And External Connections Warnings
It is the user’s responsibility to use only approved supplies, accessories and internal parts available through Mortara Instrument, Inc. Product performance and patient safety require the use of supplies, accessories and internal parts that comply with applicable standards. To maintain designed operator and patient safety, peripheral equipment and accessories used that can come in direct patient contact must be in compliance with applicable standards including IEC 60601-1, or other IEC standards (e.g., IEC 60950) as appropriate to the... -
Page 15: Use With Electro Surgery Devices Warnings
A VESA-standard adapter is available on the back of the Surveyor system for wall, swivel-arm or rolling-stand mounting. The user is responsible for correct installation of the system. Do not mount the S12 on a rolling stand at a height exceeding 110 cm (43”). The S19 should NOT be mounted on a rolling stand. -
Page 16: Ecg Calculated Heart Rate Warnings
However, the data should not be used as a sole means for determining a patient’s diagnosis. The system is equipped with Mortara’s VERITAS™ 12-lead resting ECG interpretation algorithm. The VERITAS ECG algorithm can provide an over-reading physician with a silent second opinion through diagnostic statements output on the ECG report. -
Page 17: Warnings For Patients With Pacemakers
USER SAFETY INFORMATION MIT Database Performance Measures Mortara QRS Detection Sensitivity % 99.94 QRS Detection Positive Predictivity % 99.87 PVC Detection Sensitivity % 95.49 PVC Detection Positive Predictivity % 97.05 PVC Detection False Positive Rate % 0.220 AHA Database Performance Measures... - Page 18 USER SAFETY INFORMATION wrapping too tightly, applying supplemental tape, failing to inspect periodically, or failing to position appropriately. Read the Instructions for Use provided with the SpO2 sensor carefully prior to use. Do not sterilize or immerse pulse oximetry sensors in liquid. Clean and/or disinfect re-usable sensors between patients.
-
Page 19: Nibp Warnings
USER SAFETY INFORMATION shivering. Nail polish and/or artificial fingernails can affect the accuracy of pulse oximetry and should be removed. Pulse rate measurement is based on the optical detection of a peripheral flow pulse. While a pulse rate does assist with the detection or absence of a peripheral pulse, the pulse oximeter should not be used as a replacement or substitute for ECG-based arrhythmia analysis. -
Page 20: Invasive Pressure Warnings
USER SAFETY INFORMATION An irregular heart beat (arrhythmia) causes beat-to-beat blood pressure variations and may therefore disturb the NIBP measurement, which may fail or be inaccurate. It is advisable to confirm automatic NIBP measurements periodically for patients with frequent premature beats or a very irregular heart rate, for example caused by atrial fibrillation. - Page 21 USER SAFETY INFORMATION Refer to the catheter package insert provided with each PA catheter for the appropriate computation constant, specific instructions on catheter placement and use, warnings, cautions, and specifications. Inaccurate Cardiac Output measurements may be caused by: Incorrect placement or position of the catheter.
-
Page 22: Cautions
Do not connect the patient monitor to any unauthorized patient monitors or use any third-party accessories. This may cause inaccurate measurements or harm the patient. Installation and connection to data networks must be performed by properly trained personnel, authorized by Mortara. ... -
Page 23: Notes
USER SAFETY INFORMATION Microstream® etCO2 sampling lines are designed for single patient use, and are not to be reprocessed. Do not attempt to clean, disinfect, sterilize or flush any part of the sampling line as this can cause damage to the monitor. - Page 24 ETL-Listed device in the USA and Canada. Upon request, Mortara can supply a Service Manual that includes additional calibration and test instructions as well as list of spare parts and accessories that must be used with the Surveyor patient monitors.
-
Page 25: Equipment Symbols And Markings
Tested for safety by the Intertek External power AC/DC power supply; use according to applicable U.S. and only Mortara Power Supply; REF 4101-012 Canadian standards and requirements Power On/Off switch Consult accompanying documents Local Area Network interface External alarm interface Interface to external devices –... - Page 26 EQUIPMENT SYMBOLS AND MARKINGS Connector for CO parameter Connector for CO parameter exhaust port Catalog number for relevant Mortara Serial number part This end up Keep away from sunlight Fragile, handle with care Keep dry Storage temperature range 10 rolls of recorder paper per case...
-
Page 27: Electromagnetic Compatability (Emc)
See Table X-4 for recommended separation distances between the radio equipment and the patient monitor. The use of accessories, transducers, and cables other than those specified by Mortara Instrument may result in increased emissions or decreased immunity of the equipment. -
Page 28: Able X-1 Guidance And Anufacturer Declaration Electromagnetic Emissions
ELECTROMAGNETIC COMPATABILITY (EMC) Table X-1 Guidance and Manufacturer’s Declaration: Electromagnetic Emissions The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or the user of the equipment should ensure that it is used in such an environment. Emissions Test Compliance Electromagnetic Environment: Guidance... -
Page 29: Able X-3 Guidance And Anufacturer Declaration Electromagnetic Immunity
ELECTROMAGNETIC COMPATABILITY (EMC) Table X-3 Guidance and Manufacturer’s Declaration: Electromagnetic Immunity The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or the user of the equipment should ensure that it is used in such an environment. IEC 60601 Test Compliance Immunity Test... -
Page 30: Equipment
ELECTROMAGNETIC COMPATABILITY (EMC) Table X-4 Recommended Separation Distances Between Portable and Mobile RF Communications Equipment and the Equipment The equipment is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the equipment can help to prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the equipment as recommended in the table below, according to the maximum output power of the communications equipment. -
Page 31: General Care And Maintenance
7. GENERAL CARE AND MAINTENANCE Precautions Power off the patient monitor before inspecting or cleaning. Protect the patient monitor from liquids. Never immerse the patient monitor in water. Do not drop the patient monitor or subject to shock and/or vibration. ... - Page 32 GENERAL CARE AND MAINTENANCE CAUTION: Keep the patient accessories off of the floor. Accessories that fall on the floor should be inspected for defects, contamination, proper functionality, and cleaned or discarded according to the approved recommendations. CAUTION: The user has the responsibility to validate any deviations from the recommended method of cleaning and disinfection.
- Page 33 GENERAL CARE AND MAINTENANCE Reusable NIBP Cuffs Approved Cleaning Agents Mild detergent and water Non-chlorine bleach Procedure Prior to washing, remove any internal cuff bladders and engage the Velcro hook and loop fasteners to prevent lint from collecting in the hooks. For general cleaning of cuffs, use a soft, lint-free cloth lightly moistened with a mild soap and water solution.
-
Page 34: Maintenance
The following table shows the recommended maintenance procedures for the Surveyor patient monitor and its accessories. The Surveyor S12 and S19 patient monitors should be serviced and calibrated once a year by a Mortara authorized service technician. However, it is good practice to periodically ensure the patient monitor is in proper working order. - Page 35 GENERAL CARE AND MAINTENANCE Functionality Procedure NOTE: Do not allow system to remain pressurized and stable below 20 mmHg. The monitor will remove this pressure as a zero offset and this will affect the validity of the calibration check. NOTE: The following are required to perform this test: NIBP simulator or sphygmomanometer along with a Y-cable and a hand inflation bulb.
- Page 36 If the monitor housing was opened for repair or inspection work, the following safety tests should be performed in accordance with the IEC 60601-1 or IEC 62353 methods and limits. The S12 and S19 are considered a Class 1 Type CF devices, intended to be utilized with the Mortara specified patient modules and product accessories.
- Page 37 GENERAL CARE AND MAINTENANCE Nurse call / Ethernet Test: The ports on the back of the device (nurse call/ethernet) are tested at a different voltage as defined below. The negative (black) connection of the tester should be connected to the DC Power Input via TF0571 (or equivalent).
- Page 38 Non-conductive (fully insulated) chassis testing should be performed utilizing 200 cm2 conductive foil or equivalent. Patient Leakage Applied part – patient input (utilize Mortara patient cable 9293-050-60 or -61) Patient Leakage (mains on applied part) Applied part – patient input (utilize Mortara patient cable 9293-050-60 or -61)
-
Page 39: Battery Replacement
CAUTION: Batteries should only be replaced by trained service personnel. To replace the battery: 1. Obtain a replacement battery from Mortara (see Accessories section for part number). 2. Power-off the Surveyor patient monitor. 3. On the back of the Surveyor patient monitor, disconnect the external power supply from the monitor. -
Page 40: Battery Conditions
GENERAL CARE AND MAINTENANCE Battery Conditions When the Surveyor S12 and S19 monitor’s battery has less than 5 minutes of power remaining, the battery icon flashes, a battery alert message displays, and an audio technical tone is sounded. When the battery power is too low to continue normal operation, the Surveyor S12 and S19 monitor’s screen clears, a Battery Nearly Depleted message displays in the center of the screen, and monitoring is discontinued. -
Page 41: Invasive Pressure Calibration
Upon successful completion, disconnect the calibration gas and hold the power button to power down the S12/19 device. It is not necessary to exit out of the calibration menus. Turn monitor on and reconnect the CO2 sampling line to the S12/19 device in preparation for verifying ETCO2 performance is within specified tolerances. -
Page 42: S12/S19 Preventative Maintenance Record
GENERAL CARE AND MAINTENANCE S12/S19 Preventative Maintenance Record Unit Serial #: □ Mechanical Integrity □ Device Cleaning Functional Testing Power LED PASS / FAIL (circle) Speaker Test PASS / FAIL (circle) Second Speaker Test PASS / FAIL (circle) ECG/Respiration PASS / FAIL... - Page 43 GENERAL CARE AND MAINTENANCE...
-
Page 44: Device Setup
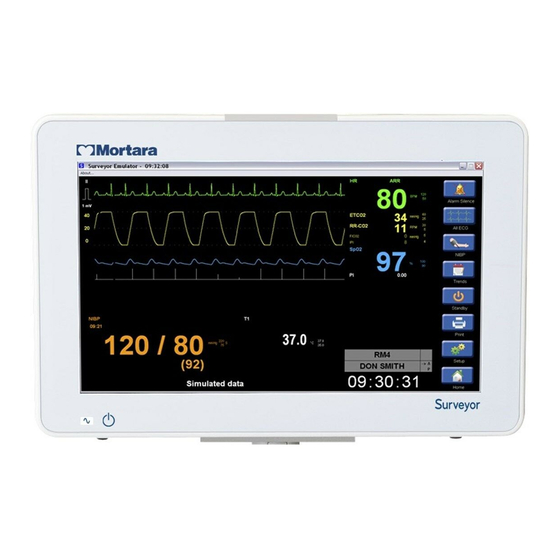
8. DEVICE SETUP Overview The Setup quick action key provides access to advanced functions such as arrhythmia settings, alarm settings, and other monitor configuration menus. Example Setup Dialogue Patient Information The Patient Information button provides access to the Patient Information dialogue. Access to this dialogue can also be obtained by selecting the black patient information area located above the system date and time. -
Page 45: Waveforms
DEVICE SETUP Example Setup Parameters Dialogue To enable/disable a parameter: 1. Select the Setup quick action key on the main screen to access the Setup dialogue. 2. Select the Parameters button to access the Setup Parameters dialogue. 3. Locate the parameter label. 4. - Page 46 DEVICE SETUP Example Setup Waveform Area Dialogue To select a waveform for display on the main screen: 1. Select the Setup quick action key on the main screen to access the Setup dialogue. 2. Select the Waveforms button to access the Setup Waveform Area dialogue. 3.
-
Page 47: Recorder
4. Select the OK button to enter that change OR select the Cancel button to cancel the changes. Recorder The Surveyor S12 and S19 patient monitors may have a two-channel thermal bedside recorder as a purchasable option. The Recorder dialogue provides configuration for which two waveforms print, the length of the recording strip, and the recording delay. -
Page 48: Arrhythmia
DEVICE SETUP To select the recording delay for the bedside recorder (optional): 1. Select the Setup quick action key on the main screen to access the Setup dialogue. 2. Select the Recorder button to access the Setup Recorder dialogue. 3. Locate the Recording Delay label. 4. -
Page 49: Alarm Suspend
DEVICE SETUP To disable all the non-lethal arrhythmias with one action: 1. Select the Setup quick action key on the main screen. 2. Select the Arrhythmia button in the Setup menu. 3. Select the Arrhythmia OFF button. 4. This action disables all the non-lethal arrhythmia alarms. 5. - Page 50 DEVICE SETUP Example Setup Alarms Dialogue To adjust numeric parameter alarm settings: 1. Select the Setup quick action key on the main screen. 2. Select the Alarms button in the Setup menu. 3. Locate the numeric parameter to adjust. Select the Next Page button to scroll to see more numeric parameters (based on monitor configuration).
-
Page 51: Audio
DEVICE SETUP Audio The Setup Audio dialogue is where the speaker volume and the HR/PR tone volume are configured. This area is also where the HR/PR systole beep tone can be enabled and disabled. Example Setup Audio Dialogue To adjust the speaker volume: 1. -
Page 52: Restore Departmental Defaults
DEVICE SETUP Restore Departmental Defaults Restore Departmental Defaults To restore the patient monitor back to the departmental default configuration: 1. Select the Setup quick action key on the main screen to access the Setup dialogue. 2. Select the Restore Departmental Defaults button. 3. -
Page 53: Configuration
DEVICE SETUP Configuration Selecting this item displays the setup of this Surveyor including its serial number, Ethernet MAC address, internal software version as well as those of accessories and modules integrated with this system. There are no configuration options here but this information is helpful for service personnel when analyzing the system for issues. Example Setup Configuration Dialogue Communications Use this dialogue to configure the communication parameters of the Surveyor including the Bed ID, Unit ID as well... -
Page 54: Screen Cleaning
DEVICE SETUP Screen Cleaning Based on the clinician’s discretion, the bedside monitor’s touch screen may require occasional cleaning. The touch screen may be cleaned with a soft, lint-free cloth and a non-abrasive, gentle cleaner such as plain soap and water. Avoid spraying cleaning agents directly onto the monitor’s touch screen. - Page 55 DEVICE SETUP Alarm Silence Time This setting controls the length of silence time for the Alarm Silence quick action key on the main screen. To select the Alarm Silence Time: 1. Select the Setup quick action key on the main screen to access the Setup dialogue. 2.
-
Page 56: Administration Setup System Dialogue
Lower limit violation delay Upper limit violation delay HR, PR (from SpO2), PR (from IBP) 3 seconds 3 seconds 5 seconds 5 seconds Mortara SpO2, Nellcor SpO2 (SatSeconds 10 seconds 10 seconds disabled) 10 seconds 0 seconds Nellcor SpO2(SatSeconds enabled), all other... -
Page 57: Administration Setup Service Dialogue
Defaults” icon and press “Ok”. You will see an on screen message that says “Please restart monitor for changes to take effect”. Power cycle the unit, and start a monitoring session with a new patient. If required, refer to S12/S19 User Manual, 9515-183-50-xxx for that process. -
Page 58: Unit Dissassembly
9. UNIT DISSASSEMBLY S12 and S19 Patient Monitors Unit disassembly and repair activities are to be performed by Authorized Mortara Service Representatives. - Page 59 UNIT DISASSEMBLY S12 Monitor Mechanical Overview...
- Page 60 UNIT DISASSEMBLY S19 Monitor Mechanical Overview...
-
Page 61: Battery Removal & Replacement
2. Locate the 4 screws (item 11) that retain the battery door (Item 45) and remove them. S19 picture above, the location of the screws on the S12 are identical. 3. Remove the battery door (Item 45) to access the battery (Item 7) 4. -
Page 62: Rear Housing Removal & Replacement
4. Locate the eight (8) mounting screws (Item 11) for the Rear Housing and remove them. (S19 shown – locations are identical for the S12) 5. Once the screws are removed, carefully lift the Rear Housing assembly straight up approx 3 to 4 inches... - Page 63 7. Once the Beacon Board cable is disconnected from the main board, the Rear Housing can be raised for easier access to the remaining cables. Note: For the S12 monitor the Beacon Board and cable will now be loose and can be removed from the LCD housing.
- Page 64 UNIT DISASSEMBLY 8. Remove the remaining cables form the circuit boards, noting the cable size and location. S19 Cable Connections S12 Cable Connections Table of Cable Connections Unit Cable Board Connection Item # S12 & S19 Main LCD (large Cable) Processor/Bitsy board connector JJ8 S12&...
-
Page 65: Processor Board Removal & Replacement
UNIT DISASSEMBLY Processor Board Removal & Replacement 1. With the battery and the Rear Housing removed locate the 2 nuts (Item 44) retaining the processor board (Item 41) to the Main Board and remove them. 2. Lift gently straight up on both ends of the processor board (Item 41) as shown to remove the PCA from the two connector sockets on either end of the board. -
Page 66: Main Board Removal & Replacement
UNIT DISASSEMBLY Main Board Removal & Replacement If the main PCBA requires replacement, the conformance testing section will need to be performed. In addition, the unit serial number will need to be entered into system memory by navigating to the following password protected factory menu screen: Setup, Administration, Factory. - Page 67 UNIT DISASSEMBLY 3. Carefully lift the main board out of the Rear Housing, there will be at least 2 cables that will need to be removed, the Battery Interconnect Cable (Item 4) and the Speaker Cable (Item 1). 4. Reach between the Rear Housing and the Main board and remove the Battery Interconnect Cable (Item 4) and Speaker Cables (Item 1).
-
Page 68: Lcd Removal & Replacement S12
LCD Removal & Replacement S12 Please note that the LCD and front bezel for the S12 comes as a complete assembly (Item 23). If the LCD requires replacement Mortara recommends replacing the entire assembly. If the LCD is OK, but the touch screen or bezel require replacement, Mortara recommends replacing the Bezel/Touchscreen assembly (Item 25). -
Page 69: Lcd Removal & Replacement S19
Please note that the LCD and front bezel for the S19 comes as a complete assembly (Item 24). If the LCD requires replacement Mortara recommends replacing the entire assembly. If the LCD is OK, but the touch screen or bezel require replacement, Mortara recommends replacing the Bezel/Touchscreen assembly (Item 26). - Page 70 UNIT DISASSEMBLY 4. Locate the 4 screws that hold the LCD to the front LCD Bezel. 5. Lift the LCD out of the Front bezel 6. Reassemble in reverse order Ensure that the cables do not get pinched when reassembling.
-
Page 71: Removal And Replacement Of The Optional Thermal Writer (S12 Only)
Removal and Replacement of the Optional Thermal Writer (S12 ONLY) The S12 has an optional single channel thermal writer (Bedside Recorder, Item 8). S12 monitors with this option have a different Rear Housing however removal of the Rear Housing removal procedure is identical. - Page 72 UNIT DISASSEMBLY 2. Remove the paper roll from the writer assembly. 3. Locate the mounting screws for the writer. Note: These screws are captive screws and they will remain attached to the writer assembly.
- Page 73 UNIT DISASSEMBLY 1. Slide the writer (Item 8) out of the Rear Housing being carful of the attached data cable. 2. Follow the writer data cable into the unit to reach the snap in RJ45 connector. Remove the cable from the Main Board by unlatching the cable form the connector.
-
Page 74: Optional Thermal Writer For The S19
UNIT DISASSEMBLY Optional Thermal Writer for the S19 The optional thermal writer for the S19 monitor comes as a completed assembly (Item 43). Service parts for this item are not sold separately. - Page 75 UNIT DISASSEMBLY The items listed in the S12/S19 Item Identification Table identify the serviceable level of the device. Subcomponents of assemblies listed are not available as individual service items from Mortara, the assembly level item must be used for servicing purposes.
- Page 76 UNIT DISASSEMBLY S12/S19 Item Identification Table Item # Part # Description Item PCB ASSY BATTERY 26025-118-50 CONNECTOR & CABLE POWER CORD US/CAN 3181-008 HOSPITAL 5-15P+320-C13 POWER SUPPLY 100-240VAC 4101-012 15VDC 40W BATTERY RECHARGEABLE 4800-017 LITHIUM ION 5450-006-50 THERMAL PRINTER 50mm ROLL...
- Page 77 UNIT DISASSEMBLY S12/S19 Item Identification Table Item # Part # Description Item SCREW THD-FORM PAN HD 6020-060 TORX 4-20x1/4" SCREW THD-FORM PAN HD 6020-065 TORX 4-20x5/16" 6020-836 SCREW PAN HD TORX M3x8 SS 6140-006 E-RING 4mm ID 6140-007 C-RING 5mm ID...
- Page 78 UNIT DISASSEMBLY S12/S19 Item Identification Table Item # Part # Description Item MODULE SpO2 EXT CABLE (Front) 6901-017-01 SENSOR NELLCOR (Back) FITTING PANEL MNT CONN 6901-019 NYLON 3/32"&1/16" (CO2 EXHAUST FITTING) PAD PROCESSOR BD 8363-033-50 SUPPORT HOUSING REAR S12 8363-001-50...
- Page 79 S12/S19 Item Identification Table Item # Part # Description Item HOUSING REAR SURVEYOR S12 8363-002-50 / S19 BEZEL S12 FINAL ASSY W/ LCD 8363-003-53 AND TOUCH BEZEL S19 FINAL ASSY W/ LCD 8363-004-53 AND TOUCH BEZEL S12 WITH TOUCH 8363-003-52...
- Page 80 S12/S19 Item Identification Table Item # Part # Description Item 8363-013-50 RETAINER CONN S12 / S19 BRACKET RECORDER MTG S12 8363-016-50 INTERNAL SPRING INPUT PANEL 8363-017-50 SPRING RETAINER ORIDION INLET DOOR ETCO2 SURVEYOR S12 / 8363-018-50 8363-024-50 WASHER SPEAKER MOUNT...
- Page 81 S12/S19 Item Identification Table Item # Part # Description Item 9042-080-03 LABEL NELLCOR OXIMAX LABEL NAMEPLATE SURV S12 9050-090-01 PATIENT MONITOR LABEL NAMEPLATE SURV S19 9050-090-02 PATIENT MONITOR PROCESSOR BOARD S12/S19 SERV-9960- (Bitzy Board) 066-03 8363-005-50 REAR COVER BEZEL S19...
- Page 82 Item # Part # Description Item 5450-007-50 External Writer Kit S19 S12/S19 PLATEN ROLLER SERV PART 183- ASSEMBLY (For use in 5450-007-50) 6150-003 NUT M3 8363-038-50 COVER BATTERY S12 / S19 VIDEO CABLE 25020-085-50 BACKLIGHT CABLE 25020-084-50 MANIFOLD ASSEMBLY NIBP 8363-029-50...
- Page 83 S12/S19 Item Identification Table Item # Part # Description Item 9042-080-01 LABEL MORTARA SpO2 9042-080-03 LABEL NELLCOR OXIMAX MUFFLER-FILTER NIBP 6301-001 PUMP INTAKE 8363-027-50 PUMP & WIRE ASSY CABLE TIE 8” 7495-018 VALVE ASSEMBLY .050 IN 8363-026-50 ORIFICE TAPE KAPTON DISC 3/8"...
- Page 84 S12/S19 Item Identification Table Item # Part # Description Item INPUT PANEL w-etCO2 8363-007-50 Printed, 4p CO INPUT PANEL w-etCO2 8363-008-50 Printed, 2p no CO INPUT PANEL wo-etCO2 8363-009-50 Printed, 4p CO INPUT PANEL wo-etCO2 8363-010-50 Printed, 2p no CO...
-
Page 85: 10. Conformance Testing
10. CONFORMANCE TESTING Conformance Testing Conformance testing is to be performed by Authorized Mortara Service Representatives to verify the device is functioning correctly after repair operations have been performed. Testing results should be documented on the test data record at the end of this section of the manual. -
Page 86: Power Testing
Once the monitor powers up and enters the main screen, the current draw (on current) should read as follows (depending on the model). Record the test result. S12 Patient Monitor <1.6A S19 Patient Monitor <2.3A Verify the battery icon appears in the lower right section of the monitor. - Page 87 CONFORMANCE TESTING 2.1.2 Plug in the Power Cord into the back of the UUT and then press the Power button to power up the UUT as shown below: In the UUT screen select “Recalibrate Touch Panel”. 2.1.3 2.1.4 Use a pen to touch the intersection of the two lines in the middle of the screen. NOTE: The software will move the lines to a new location.
- Page 88 CONFORMANCE TESTING In the UUT select the “Save Touch Panel Cal” button after the touch screen calibration is 2.1.5 complete as shown below: 2.2 Monitor Brightness Adjustment Check On the UUT display, tap the “Dimmer Display” button to verify the screen changes 2.2.1 accordingly.
- Page 89 CONFORMANCE TESTING 2.2.3 On the UUT press and hold the power button to turn off the UUT. 2.2.4 On the UUT, remove TF-0564 USB test drive.
- Page 90 CONFORMANCE TESTING 2.3 Speaker Volume and Date/Time Verify 2.3.1 Insert TF-0563 USB Production Dongle. 2.3.2 Power on UUT by pressing the power button. Select “No” to the New Patient screen when UUT powers on. 2.3.3 2.3.4 Perform Audio Check.
- Page 91 CONFORMANCE TESTING Select "Setup” button from lower right of the UUT. Select “Audio”. Run a speaker volume and HR/ PR Tone volume check: pressing the Up/Down arrows while verifying the volume increases as the numbers get higher, and decreases as the numbers get smaller (1-9). Then Press "OK”...
- Page 92 CONFORMANCE TESTING 2.4.3 On the UUT go to Setup>Administration>Communications to open the “Communications” menu and change the Use DHCP box to “Yes”. Then press “OK”. The UUT will reboot. 2.4.4 On the UUT go to Setup>Administration>Communications to open the “Communications” menu. Verify the Ethernet connection IP Address has been populated with an IP address other than 0.0.0.0.
- Page 93 CONFORMANCE TESTING 2.5.3 Verify a full test strip prints including: a patient information section, strip chart report section, and parameter snapshot section. 2.6 Nurse Call Functional Check 2.6.1 Insert TF-0565 nurse call test fixture into the right side panel of the UUT next to the Bell Icon. 2.6.2 Open the Recorder Door and verify the door open alarm sounds.
- Page 94 CONFORMANCE TESTING 2.7 NIBP Testing NOTE: Do not allow system to remain pressurized and stable below 20 mmHg. The monitor will remove this pressure as a zero offset and this will affect the validity of the calibration check. NOTE: The following are required to perform this test: NIBP simulator or sphygmomanometer along with a Y-cable and an NIBP cuff.
- Page 95 CONFORMANCE TESTING 2.8 ECG Input Test 2.8.1 Remove the USB production dongle TF-0563 from the UUT. 2.8.2 Connect the AM12M USB cable to the UUT port. Verify the 12 patient leads are connected to the EL400 simulator. 2.8.3 From the main screen of the UUT, touch the HR menu in the upper right corner (usually displaying the heart rate reading to open the Setup HR/PR menu.
- Page 96 CONFORMANCE TESTING Select “OK”. 2.8.5 From the main screen select “12 Lead ECG”. 2.8.6 2.8.7 Verify that all 12 ECG waveforms are displayed and show no signs of distortion.
- Page 97 CONFORMANCE TESTING Select “Close” to return to the main screen. 2.8.8 2.8.9 From the main screen, verify the UUT reads 60 +/- 1 bpm. Record the test result. 2.8.10 Disconnect the AM12M from the UUT and verify a Lead Off alarm sounds. Reconnect the AM12M USB to the UUT.
- Page 98 CONFORMANCE TESTING In the Setup HR/PR menu set the 12-Lead Enabled setting to “NO”. 2.8.12 Select “OK”. 2.8.13 The Lead Off alarm will sound. Select the “Alarm Silence” icon to turn off the alarm. 2.8.14 2.8.15 Disconnect the AM12M USB cable from the UUT.
- Page 99 CONFORMANCE TESTING 2.8.16 Connect the green ECG (5) lead patient cable to the appropriate side port. Verify the 5 patient leads are connected to the EL400 simulator. 2.8.17 Verify on the UUT main screen that the ECG waveform and the RR-ECG waveform appear. Note: If the UUT does not display the RR-ECG waveform, select Setup>Parameters and set ETCO2 Enabled to “NO”.
- Page 100 CONFORMANCE TESTING From the main screen, select “All ECG”. 2.8.19 2.8.20 Verify the UUT displays Leads II, V, and I and no traces show any sign of distortion. Note: If the UUT only displays Lead I, select the “HR” in the top right corner to enter the Setup HR/PR menu.
- Page 101 CONFORMANCE TESTING Set the patient simulator EL400 to input a Paced Beat. On the simulator press “ECG”. Go to 2.8.22 the Wave Group: NSR (Adult) setting press Enter and scroll down to the TV Paced wave group. Press Enter to select this wave group. Verify that white pace marks appear above Pacemaker Spikes on the ECG display of the UUT.
- Page 102 CONFORMANCE TESTING 2.8.25 Reconnect the 5 Lead ECG cable to the UUT and turn on the patient simulator. Select the “Normal ECG” button to return to the main screen.
- Page 103 Reset the Rate to 20 brpm. Remove the 5 Lead ECG cable from the UUT and turn off the “Lead Off” alarm using the “Silence Alarm” button. 2.10 SpO2 Check 2.10.1 Connect the appropriate SpO2 cable (Mortara or Nelcor) to the UUT port. 2.10.2 Verify that the RED LED light is ON in the finger clip.
- Page 104 CONFORMANCE TESTING On the EL400 simulator press the “SpO2” button. Verify that the SpO2 2.10.3 Test Value is set to 96%. (Note: Bottom signal bar on the simulator screen is blank) 2.10.4 Connect the SpO2 Finger Clip to the EL400 patient simulator. (Note: the signal bar on the simulator should be green.
- Page 105 CONFORMANCE TESTING Connect TF-0560 IBP “Y” cable to the UUT IBP side panel connection. 2.11.2 The UUT will show flat lines on the 2 IBP channels and alarm “P1 and P2 Needs 2.11.3 Calibration”. (Note: If the waveforms are not present select Setup>Parameters>IBP Channels and then select 2 (or 4 if 4 IBP channels are present) from the drop down menu.
- Page 106 CONFORMANCE TESTING 2.11.4 On the EL400 patient simulator press the “IBP” button. (NOTE: If the Screen displayed as i below, change the setting to ii below). Channel 1 Chamber: Arterial Pressure: 120/80 mmHg Artifact: Off Channel 2 Chamber: Arterial Pressure: 120/80 mmHg Artifact: Off Press “F1 &...
- Page 107 CONFORMANCE TESTING Verify “PX Calibration OK” shows at the bottom of the UUT display. iii. Perform this calibration step for all available PX channels. On the UUT, select OK>OK>Close>Close to return to the main screen. 2.11.6 Zero the PX channels. On the UUT, select one of the PX readings to open the Setup PX menu.
- Page 108 CONFORMANCE TESTING Verify the “PX Zero OK” message appears at the bottom of the UUT display. iii. Verify that both PX channels read 0/0 mmHg. 2.11.7 On the EL400 patient simulator press the “IBP” button. Set channel 1 & 2 to the settings displayed below.
- Page 109 CONFORMANCE TESTING 2.11.9 For IBP channels 3 & 4 repeat testing from step 2.11.4 for those 2 IBP channels with TF-0560 connected to P3 & 4 port. 2.11.10 Once testing is complete on all channels, disconnect TF-0560. Verify UUT alarms and displays “PX unplugged”.
- Page 110 CONFORMANCE TESTING 2.12.3 Verify the UUT T1 reading is 37° +/- 1° C. (Note: If the T1 and T2 reading does not appear on the UUT display, select Setup>Parameters and set the Temperature Display Mode to T1 and T2 from the drop down menu. Press “OK” and “Close”...
- Page 111 CONFORMANCE TESTING 2.15.6 Disconnect the TF-0561 temperature cable from T2 of the UUT. Verify the UUT alarms and displays “T1 unplugged”. Press the “Alarm Silence” button to turn off the alarm.
- Page 112 CONFORMANCE TESTING 2.13 Cardiac Output 2.13.1 Connect TF-0562 CO cable to the Cardiac Output of the EL400 patient simulator. 2.13.2 Connect the other end of the TF-0562 CO cable to the CO port of the UUT. On the UUT, select the CO reading on the display to open the “Setup CO” menu. 2.13.3 (Note: If the UUT does not display CO and CI select Setup>Parameters and then set the CO Enabled setting to YES.
- Page 113 CONFORMANCE TESTING 2.13.16 In the Measure CO menu, set the Mode to Manual from the drop down menu. 2.13.17 Adjust the TF-0562 dial so that the IT reading on the UUT Measure CO menu reads 10 +/- 0.2. Verify the UUT BT reading shows 37 +/- 2. 2.13.18 On the UUT, press “Start”...
-
Page 114: Device Cleaning
CONFORMANCE TESTING Disconnect the CO2 Filter Line Set from the UUT. Verify the UUT alarms and shows “CO2 2.14.4 unplugged” on the display. Verify the LED alarm on the UUT lights up. On the UUT, select the “Alarm Silence” button to turn off the alarm. Press and hold the 2.14.5 Power Button to turn off the UUT. -
Page 115: Safety Testing
If the monitor housing was opened for repair or inspection work, the following safety tests should be performed in accordance with the IEC 60601-1 or IEC 62353 methods and limits. The S12 and S19 are considered a Class 1 Type CF devices, intended to be utilized with the Mortara specified patient modules and product accessories. - Page 116 CONFORMANCE TESTING Nurse call / Ethernet Test: The ports on the back of the device (nurse call/ethernet) are tested at a different voltage as defined below. The negative (black) connection of the tester should be connected to the DC Power Input via TF0571 (or equivalent).
- Page 117 Non-conductive (fully insulated) chassis testing should be performed utilizing 200 cm2 conductive foil or equivalent. Patient Leakage Applied part – patient input (utilize Mortara patient cable 9293-050-60 or -61) Patient Leakage (mains on applied part) Applied part – patient input (utilize Mortara patient cable 9293-050-60 or -61)
- Page 118 PASS / FAIL (Circle) □ Off Current uA (< 350uA) PASS / FAIL □ On Current A (S12 < 1.6A, S19 < 2.3A) PASS / FAIL □ Charging Current / AC Power LED PASS / FAIL Functional Testing Complete Test...
-
Page 119: 11. Product Specifications
11. PRODUCT SPECIFICATIONS General Specifications S12: 315 W x 203 H x 125 D mm (12.4 W x 8.0 H x 4.9 D inches) Dimensions S19: 468 W x 289 H x 97 D mm (18.4 W x 11.4 H x 3.8 D inches) S12: 3 Kg (6.6 lbs) -
Page 120: Power Requirements & Battery
High definition, antiglare 16:9 Color TFT-LCD with LED backlight and resistive touch Type panel controls S12: 11.6 inches diagonal; 256mm x 144 mm active area; 1366 x 768 pixels Size & Resolution S19: 18.5 inches diagonal; 410mm x 230 mm active area; 1366 x 768 pixels... -
Page 121: Mounting Specifications
Conforms to MIS-D 75 (75 mm × 75 mm) and MIS-D 100 (100 mm × 100 mm) standards for optional mounting on a rolling stand (max. height 100 cm or 43 inches), wall mount or articulating wall mount using Mortara specified accessories. -
Page 122: Parameter Specifications
Waveform Sweep Speed 6.25 ms, 12.5 ms, 25 ms & 50 ms Waveform Delay 0.5 seconds on all parameters 3/5 Lead Cable or Mortara AM12M 12-Lead ECG Acquisition Module ECG Interpretation Available with AM12M only ECG Modes Adult / Pediatric... -
Page 123: Arrhythmia Analysis
PARAMETER SPECIFICATIONS Beat Recognition: Normal, Ventricular, Paced, Unknown The algorithm calculates the heart rate from its available source. If the heart rate from the last four R to R intervals is greater than 48 beats per minute, the average heart rate is determined by averaging the last 16 R to R intervals. HR Averaging If the heart rate from the last four R to R intervals is less than or equal to 48 beats per minute, then this rate is used. -
Page 124: St Analysis
PARAMETER SPECIFICATIONS processing are selected internally from the set II and V5 for detection, and V1 for confirmation. 3 lead: Displayed ECG vector 5 lead: When configured to Auto, the ECG leads used for HR and ARR processing start with II and V for detection and III for confirmation while allowing the algorithm to switch to other available leads based on signal quality. -
Page 125: Non-Invasive Blood Pressure (Nibp)
(Functional oxygen saturation of arterial hemoglobin) Parameters Plethysmogram (trace), % SpO2, Pulse rate : 1% O Resolution PR: 1 bpm (beat per minute) SpO2: 30 – 100 %, calibrated range 70-100% PR: 30 – 240 bpm (Mortara) Measurement Range PR: 25 – 250 bpm (Nellcor) -
Page 126: Temperature
SpO2: from 70 to 100%: ±2% (O2%), < 70%: unspecified Measurement Accuracy PR: ±3 bpm Per ISO9919 Clause 50 Measurement Test Method (Mortara SpO2 A = 1.73%) (Nellcor SpO2 A = 1.9%) Report Interval 1 second. Numeric values held < 30 seconds... -
Page 127: Invasive Pressures
PARAMETER SPECIFICATIONS EtCO2 + FiCO2: 1 mmHg Measurement Resolution Respiration: 1 bpm (breath per minute) etCO2: + FiCO2: 0 to 150 mmHg Measurement Range Respiration: 0 to 150 bpm EtCO2: + FiCO2: 0 to 38 mmHg: ± 2 mmHg 38 to 150 mmHg: ± (5% or reading + 0.08% for every 1 mmHg > 38 mmHg) Accuracy applies for breath rates of up to 80 bpm. -
Page 128: Cardiac Output
PARAMETER SPECIFICATIONS Resolution: 1 mmHg Measurement Range -50 to 300 mmHg Measurement Accuracy ±1 mmHg or ±1%, whichever is greater Report Interval Every 3 seconds 30 – 250 bpm PR Range PR Accuracy ±2 bpm or ±2%, whichever is greater PR Resolution 1 bpm 0 –... - Page 129 PARAMETER SPECIFICATIONS Computation Constant 0.001 Resolution Computation Constant Range 0.000 to 0.999 CO, CI, BSA, SV, SVI, SVR, SVRI, PVR, PVRI, LVSW, LVSWI, RCW, RCWI, RVSW, Hemodynamic Calculations: RVSWI, PAWP...
-
Page 130: 13. Parameter Alarm Limit Ranges
13. PARAMETER ALARM LIMIT RANGES The high and low alarm limit ranges are as per the tables below. Adult Patient Mode Lower Limit Upper Limit Alarms On Alarm Level Print on Alarm Parameter Range Range Choices (Factory Default) Choices (Factory Default) (Factory Default) (Factory Default) Off, 20 –... - Page 131 PARAMETER ALARM LIMIT RANGES Lower Limit Upper Limit Alarms On Print on Alarm Parameter Alarm Level Range Range Choices Choices Off, -30 – 298 (*75) -28 – 300, Off (*220) P1 Systolic No, *Yes No* , Yes *Med Off, -30 – 298 (*50) -28 –...
-
Page 132: Pediatric Patient Mode
PARAMETER ALARM LIMIT RANGES Pediatric Patient Mode Upper Limit Alarms On Print on Alarm Parameter Lower Limit Range Alarm Level Range Choices Choices Off, 20 – 100 (50) 50 – 250, Off (150) Heart Rate *Med No, *Yes ) No* , Yes Off, Low, 10 –... - Page 133 PARAMETER ALARM LIMIT RANGES Upper Limit Alarms On Print on Alarm Parameter Lower Limit Range Alarm Level Range Choices Choices Off, -30 – 298 (75) -28 – 300, Off (145) P1 Systolic *Med No, *Yes No* , Yes Off, -30 – 298 (50) -28 –...
-
Page 134: 14. Alarm Specifications
14. ALARM SPECIFICATIONS General Alarms Audible & visible alarm indication + external nurse call interface Alarms Alarm levels: High, Medium & Low Alarm Volume: 45 - 85 dB(A) Color coded: Red, Yellow, Cyan Visual Alarm Light Complies with IEC60601-1-8 High, medium, low level, no sound Clinician adjustable volume control from 1 to 10 (45 –... -
Page 135: Non-Invasive Blood Pressure (Nibp) Messages
Dried out electrode Check to make sure there are no broken lead wires. Inoperable ECG circuit Turn monitor off, then back on If message persists, contact Mortara technical support. Calm the patient. Patient movement Isolate the patient from auxiliary equipment, if possible. - Page 136 Check the patient and insure that the cuff is deflated Monitor has detected a NIBP needs service Turn the monitor off, then on. hardware problem If message persists, contact Mortara technical support. Initial inflation pressure may not have been high Repeat the measurement (monitor will automatically enough (if patient’s...
-
Page 137: Pulse Oximetry (Spo2) Messages
Bad SpO2 sensor Replace the SpO2 sensor. Incorrect set-up within SpO2 replace sensor the Surveyor patient Contact Mortara Technical Support. monitor. Sensor has become Check to make sure the sensor is attached fully and detached from patient securely to the patient... -
Page 138: Temperature Messages
ALARM SPECIFICATIONS Check the patient and provide any necessary clinical The patient's oxygen care saturation has fallen SpO2 < [lower limit] [number] below the current lower Change the alarm limit if it is no longer clinically alarm limit. appropriate Check the patient and provide any necessary clinical The patient's oxygen care saturation has risen... -
Page 139: Respiration Messages
Inoperable respiration Check to make sure there are no broken lead wires. detection circuit Turn monitor off, then back on If message persists, contact Mortara technical support. Calm the patient. Patient movement Isolate the patient from auxiliary equipment, if possible. -
Page 140: Capnography (Co2) Messages
ALARM SPECIFICATIONS Capnography (CO2) Messages Parameter Message Possible Causes Suggested Actions Value Check the patient and provide any necessary clinical The patient's ETCO2 care. ETCO2 < [lower parameter value has [number] limit] fallen below the current Change the alarm limit if it is no longer clinically lower alarm limit. -
Page 141: Invasive Pressure Messages
ALARM SPECIFICATIONS Verify that the correct patient mode (Adult/Pediatrics) Note: Changing The patient mode has is being applied. Save or Purge patient monitoring patient mode will changed. data as appropriate, or cancel selection. clear IPI trend data, The patient age has even if other patient Verify that the patient age is correctly entered. -
Page 142: Cardiac Output Messages
IBP channel calibration Calibrating Wait until the calibration process is completed is in progress Unable to calibrate Calibration failed Contact Mortara Technical Support ART: Check ART IBP pressure Check ART catheter to ensure that it is properly Transducer below 10mmHg positioned and connected. -
Page 143: 15. Troubleshooting
15. TROUBLESHOOTING The following table provides guidance for investigating issues that may occur during operation of the Surveyor patient monitors. Contact Mortara Technical Service for further assistance. Power and Battery Symptom Possible Causes Suggested Resolution Power cycle the Surveyor patient monitor and try The Surveyor patient again. -
Page 144: Ecg, Arrhythmia, And St
Cuff selection or placement, or measurement cycle; confirm appropriate initial inflation pressure settings. Re-attempt the inaccurate. patient movement measurement. If problems persist, contact Mortara Technical Support. No action. This is as per design. The NIBP End Tone Successful completion of NIBP Audible beep heard... -
Page 145: Pulse Oximetry (Spo2)
TROUBLESHOOTING Improper cuff size or placement Ensure proper BP cuff size and placement BP values higher or lower Patient moving during BP Use comfort measures to encourage patient to keep than clinically anticipated acquisition NIBP limb still Pulse Oximetry (SpO2) Symptom Possible Causes Suggested Resolution... -
Page 146: Invasive Pressures
TROUBLESHOOTING Invasive Pressures Symptom Possible Causes Suggested Resolution Ensure the pressure manifold is properly connected, Improper manifold setup the invasive cable is inserted into the correct invasive Loose pressure tubing connections pressure channel of the patient monitor, and the Pressure cable not connected into transducer is intact. - Page 147 TROUBLESHOOTING Surveyor S12 and S19 Software Block Diagram...
- Page 148 TROUBLESHOOTING Surveyor S12 and S19 Hardware Block Diagram...
- Page 149 TROUBLESHOOTING Serial Number and Revision Information To access the “Setup Configuration” screen press Setup, Administration, Configuration. Field Name Definition Serial Number Factory Serial Number of the Patient Monitor Ethernet MAC MAC Address of the onboard LAN Main is the program that drives the Surveyor device (e.g., user interface, signal processing). It is loaded at start-up from the SD Card by the WinCE OS and runs on the Processor Board.
-
Page 150: 16. Mounting Accessories
16. MOUNTING ACCESSORIES Quick Disconnect (M-Series) Wall Mounting Components Value (Vesa M-Series) Wall Mounting Components... -
Page 151: Premium (Vhm-25) Wall Mount Components
MOUNTING ACCESSORIES Premium (VHM-25) Wall Mount Components... -
Page 152: Surveyor S12 Roll Stand Components (Not To Be Used With S19)
MOUNTING ACCESSORIES Surveyor S12 Roll Stand Components (not to be used with S19) - Page 153 MOUNTING ACCESSORIES...


Need help?
Do you have a question about the S12 and is the answer not in the manual?
Questions and answers