Aerogen Solo System Instruction Manual
Usb
Hide thumbs
Also See for Solo:
- Instruction manual (40 pages) ,
- System instruction manual (38 pages) ,
- User manual (9 pages)
Subscribe to Our Youtube Channel
Summary of Contents for Aerogen Solo
- Page 1 System Instruction Manual for use with Aerogen Solo ® and Aerogen ® www.aerogen.com...
-
Page 3: Table Of Contents
Contents Introduction Intended Use Set Up System Warnings Controls & Indicators Accessories Functional Test Aerogen Solo Aerosol Flow Rate Calculation Cleaning of the Aerogen USB Controller System Troubleshooting Warranty Life Of Products Specifications Aerogen Solo Performance Aerogen Pro Performance Power... -
Page 4: Introduction
The Aerogen Pro is suitable for use in adult, paediatric and neonate patients. The Aerogen Solo belongs to the Aerogen Pro family, the Aerogen Solo ®... - Page 5 The Aerogen USB Controller can be used with Aerogen nebulisers as follows: Table 1. Intended Use Summary Aerogen Solo Aerogen Pro Intended Use Summary Nebuliser Nebuliser Hospital - Ventilated patients Hospital -Spontaneously Breathing Patients Homecare - Ventilated patients Homecare - Spontaneously Breathing Patient...
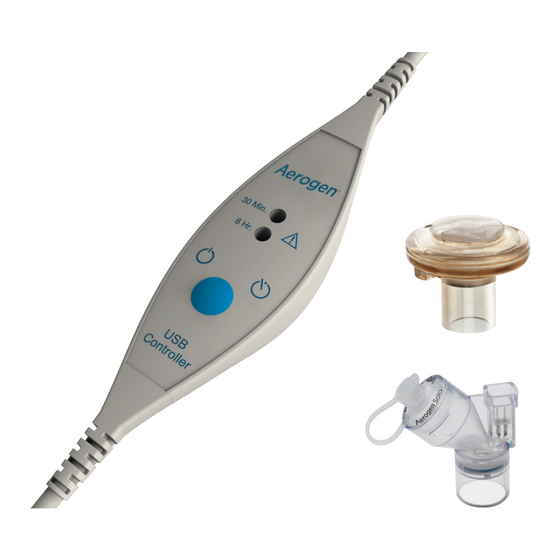
- Page 6 Aerogen Solo Figure 1. Aerogen USB Controller System (Items Provided) 1. Aerogen USB Controller 2. Aerogen Nebuliser (Aerogen Solo or Aerogen Pro) 3. T-Piece & Silicone Plug 4. Cable Management Clips 5. Aerogen USB Controller AC/DC Adapter Visit www.aerogen.com for full parts list.
-
Page 7: Set Up
Set Up Read and study all instructions before using the Aerogen USB Controller. Perform a functional test of the Aerogen nebuliser prior to use as described in the Functional Test section of this manual (see page 20). Connect Aerogen Solo Aerogen Pro nebuliser by firmly pushing into the T-piece. - Page 8 Alternative Set Up: The Aerogen Solo can be placed on the dry side of the humidifier. Connect the Aerogen USB Controller into the USB Port. Note: The Aerogen USB Controller can only be operated from a USB port medical electrical...
- Page 9 30 Min. 30 Min. 6 Hr. 6 Hr. Note: Verify the correct mode of operation is selected. Controller Controller Verify that aerosol is visible. Note: Clips are provided to assist with cable management. Aerogen USB Controller System Instruction Manual ®...
-
Page 10: System Warnings
Do not place the Aerogen USB Controller in an incubator during use. To avoid exhaled medication affecting the ventilator, follow ventilator manufacturer’s recommendations for use of a bacterial filter in the expiratory limb of a breathing circuit. - Page 11 Portable and mobile radio frequency (“RF”) communication devices can disrupt medical electrical equipment. The Aerogen Solo is a single patient use device not to be used on more than one patient to prevent cross infection. Aerogen USB Controller System Instruction Manual ®...
- Page 12 Do not obstruct the removal of the Aerogen USB Controller AC/DC Adapter from the mains. Do not store the Aerogen USB Controller System in a location where it is exposed to direct sunlight, extreme heat or cold, dust or moisture. Store out of reach of children.
- Page 13 Figure 2. Aerogen Palladium Vibrating Mesh Technology Use of the Aerogen Solo and T-piece during the administration of volatile anaesthetics may result in adverse effects on the constituent plastics. Do not use with volatile anaesthetics unless known to be compatible. Aerogen...
-
Page 14: Controls & Indicators
30 Min. 6 Hr. 6 Hour Mode Indicator light On/Off Control Controller Figure 3. Aerogen USB Controller Controls & Indicators Table 2. Aerogen USB Controller Controls & Indicators Control / Indicator Function • Green = 30 Minute nebulisation cycle on. -
Page 15: Accessories
Dry Side of the Humidifier The Aerogen Solo can be placed on the dry side of the humidifier as shown. The Aerogen Solo can be used with a nasal interface in this configuration. The Aerogen Pro is not recommended for use on the dry side of the humidifier. - Page 16 Note: To ensure correct nebulisation, maintain nebuliser vertical orientation. Use With A Nasal Interface The Aerogen Solo can be used on/off ventilator with a nasal interface when configured with a humidifier. Aerogen ®...
- Page 17 Ultra ® (Hospital Use Only) The Aerogen Ultra is an accessory specific to the Aerogen Solo nebuliser. It facilitates intermittent and continuous nebulisation and optional supply of supplemental oxygen to paediatric and adult patients via mouthpiece. The device can alternatively be used with the I-Guard Aerosol Mask, as ™...
- Page 18 1. Insert Aerogen Solo nebuliser firmly into Aerogen Ultra in orientation shown in Figure 4. 2. If supplemental oxygen is required, firmly attach oxygen tubing to Aerogen Ultra. Note: Oxygen flow rate should be set between 1-6 LPM. 3. If a face mask is required, remove mouthpiece and attach face mask to Aerogen Ultra.
- Page 19 Tubing (Syringe End) Figure 5. Continuous Nebulization Tube Set 1. Ensure the Aerogen Solo nebuliser is firmly fitted into the Aerogen Solo T-piece in the breathing circuit. 2. Remove the syringe cap from the medication-filled syringe. 3. Attach the syringe end of the tubing onto the syringe.
- Page 20 Note: Aerogen’s recommended input rate of medication into the Aerogen Solo nebuliser during continuous nebulisation is up to a maximum of 12 mL per hour. The upper limit of 12 mL per hour is based on Aerogen’s specification for the minimum nebuliser flow rate. For directions on determining flow rates, refer to the Optional Flow Rate Calculation method in the Functional Test section, page 21.
- Page 21 • Ensure that the tubing is safely orientated to prevent a trip hazard. • Rising level of medication in the reservoir may occur if the Aerogen Solo nebuliser is turned off while the feed system is still on or the nebuliser is not in its recommended orientation.
-
Page 22: Functional Test
5. Disconnect the nebuliser from the controller. Verify that the amber Error Indicator lights. Reconnect the nebuliser to the controller. 6. If using the Aerogen Solo press the On/Off power button again to turn the system off. Press and hold the button for at least 3 seconds. Verify that the 6 Hour Mode indicator light is green and that aerosol is visible. -
Page 23: Aerogen Solo Aerosol Flow Rate Calculation
Aerogen Solo. Flow rates may vary between individual Aerogen Solo nebulisers. The minimum flow rate for all Aerogen Solo nebulisers is 0.2 mL per minute. In order to calculate the flow rate of an individual Aerogen Solo nebuliser; follow these steps: 1. -
Page 24: Cleaning Of The Aerogen Usb Controller System
2. Check for exposed wiring, damaged connectors, or other defects and replace if any are visible. 3. Visually inspect for damage and replace the Aerogen USB Controller if any damage is observed. Note: Do not spray liquid directly onto the Aerogen USB Controller. - Page 25 Warning: Do not use abrasive or sharp tools to clean the nebuliser unit. Disinfection of the Aerogen Pro & Accessories Automated Washing Cycle The Aerogen Pro Nebuliser has been qualified for the following automated washing cycles. Automated Cycle One Detergent: Liquid alkaline cleaner (diluted as per manufacturers instruction).
- Page 26 4. Rinse at 90 °C (194 °F) for 1 minute. 5. Drain the machine for 40 seconds. 6. Rinse at 90 °C (194 °F) for 1 minute. 7. Drain the machine for 40 seconds. 8. Dry at 90 °C (194 °F) for 15 minutes. Aerogen ®...
- Page 27 Leave the nebuliser immersed in the boiling water for a maximum of 20 minutes. 5. Carefully remove the Aerogen Pro from the boiling water and shake off the excess water. Allow parts to fully air dry on a clean, dry towel, out of the reach of children.
- Page 28 Sterilisation of the Aerogen Pro Sterilisation of Aerogen Pro Nebuliser, T-Pieces & Neonate Adapters 1. Disconnect the nebuliser from the Aerogen USB Controller, and then remove the nebuliser and Adapters from the ventilator circuit, mask or mouthpiece.
- Page 29 100S Sterilisation System for specific instructions regarding its correct operation. Prior to next use: 1. Check for cracks or damage and replace if any defects are visible. 2. Perform a functional test as described in this manual. Aerogen USB Controller System Instruction Manual ®...
-
Page 30: Troubleshooting
Troubleshooting If these suggestions do not correct the problem, discontinue use of any device and contact your local Aerogen sales representative. Table 4. Aerogen USB Controller System Troubleshooting If this happens: It could mean: Try this: No medication in nebuliser. -
Page 31: Warranty
The Aerogen Solo nebuliser has been qualified for: • Intermittent use for a maximum of 28 days (4 treatments per day.) • For continuous use, the life of the Aerogen Solo nebuliser and the Continuous Nebulization Tube Set have been validated for use for a maximum of 7 days. -
Page 32: Specifications
Specifications Table 5. Physical Specification of the Aerogen Solo Nebuliser Dimensions 67 mm H x 48 mm W x 25 mm D (2.6 in. H x 1.88 in. W x 1 in. D) Nebuliser Weight 13.5 g (0.5 oz) nebuliser and plug... -
Page 33: Aerogen Solo Performance
Performance Table 9. Performance Specifications of the Aerogen Solo Flow Rate >0.2 mL/min (Average: ≈ 0.38 mL/min) As measured with the Andersen Cascade Impactor: • Specification Range: 1-5 μm • Average Tested: 3.1 μm As measured with the Marple 298 Cascade Impactor: •... -
Page 34: Aerogen Pro Performance
Table 10. Performance Specifications of the Aerogen Pro Flow Rate >0.2 mL/min (Average: ≈ 0.4 mL/min) As measured with the Andersen Cascade Impactor: • Specification Range: 1-5 μm • Average Tested: 3.1 μm As measured with the Marple 298 Cascade Impactor: •... -
Page 35: Power
Power Source: The Aerogen USB Controller can operate from an AC/DC Adapter (input 100 to 240 VAC 50 – 60 Hz, output 5 V) Note: The Aerogen USB Controller is approved for use with Aerogen USB Controller AC/DC Adapter AG-UC1040-XX* (Manufacturer Reference:... -
Page 36: Symbols
Symbols Table 11. Aerogen USB Controller System Symbols Symbol Meaning Symbol Meaning Serial number designation, where Timer selection (to YY is the year of select the 30 minute YYXXXXX manufacture and or 6 hour nebulisation XXXXX is the serial cycle). -
Page 37: Appendix 1: Emc Tables
Guidance and manufacturer’s declaration – electromagnetic emissions The Aerogen USB Controller system is intended for use in the electromagnetic environment specified below. The customer or the user of the Aerogen USB Controller system should assure that it is used in such an environment. - Page 38 Recommended separation distances between portable and mobile RF communication equipment and the Aerogen USB Controller system that is not life supporting The Aerogen USB Controller system is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user...
- Page 39 Aerogen USB Controller system that is not life supporting The Aerogen USB Controller system is intended for use in the electromagnetic environment specified below. The customer or the user of the Aerogen USB Controller system should assure that it is used in such an environment.
- Page 40 RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Aerogen USB Controller system is used exceeds the applicable RF compliance level above, the Aerogen USB Controller system should be observed to verify normal operation.
- Page 42 INTL. +353 91 540 400 info@aerogen.com www.aerogen.com twitter.com/aerogen Manufacturer Aerogen Ltd. © 2017 Aerogen Ltd. Part No. AG-UC1050-EN Galway Business Park, P/N 30-763 Rev D Dangan, Galway, Ireland.







Need help?
Do you have a question about the Solo and is the answer not in the manual?
Questions and answers