Summary of Contents for Omron Nami Cat
- Page 1 New Product Information Sheet Specifications subject to change © OMRON HEALTHCARE EUROPE B.V. Model (code): Nami Cat (NE-C303K-KDE) Versions Remark Date 29/04/2020 Full version, with key visual 02/07/2020 PM-1840-01-02/2020...
- Page 2 PM-1840-01-02/2020...
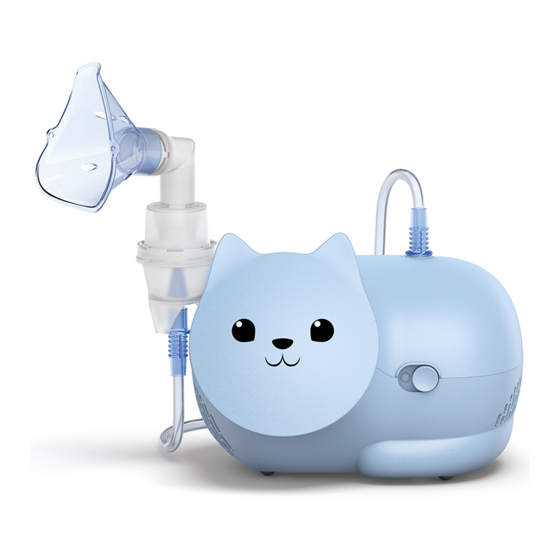
- Page 3 Nami Cat Cute outside. Powerful inside. Product Name: Nami Cat (NE-C303K-KDE) Product Description: Compressor Nebulizer Product Category: Aerosoltherapy Equipment Features Packaging content - Specially designed for children - Compressor - Efficiently delivers medication into - Nebulizer Kit the lungs - Adult MASK (PVC)
- Page 4 Product description: Compressor Nebulizer Product Category: Aerosoltherapy Equipment Model (code): Nami Cat (NE-C303K-KDE) Rating: 230V ~50Hz Power Consumption: 150VA Operating Mode: Continuous use Operating conditions: +5°C to +40°C / 15% to 85%RH / 700 to 1060 hPa Storage / Transport conditions: -20°C to +60°C / 5% to 95%RH / 500 to 1060 hPa...
-
Page 5: Application Field
General description OMRON is introducing the Nami Cat, a new addition to its Compressor Nebulizer portfolio. This product was developed in conjunction with respiratory therapists for the successful treatment of respiratory disorders such as asthma, chronic bronchitis, allergies, etc. This is a medical device. Operate the device only as instructed by your doctor and/or respiratory therapist. - Page 6 Intended User Persons who suffer from respiratory disorders and require inhalation of medications. Before using this device please refer to “Important Safety Instructions” section of the Instruction Manual. Indications for use This product is intended to be used for inhaling medication for respiratory disorders.
- Page 7 4. Add the correct amount of prescribed medication into the medication tank. 5. Verify the presence of the vaporiser head inside the medication tank. 6. Put the inhalation top back onto the medication tank. 7. Attach the adapter, and the mask or mouthpiece to the nebulizer kit tightly.
- Page 8 Materials used in main device Colorant Grade Colorant Part’s name Material grade Housing, air ABS (acrylonitrile ESTADIENE Pantone filter cover and Blue butadiene styrene) 1640 278 U switch Geotess Air Filter PP (polypropylene) White RAL 9010 EPDM (Ethylene- Legs Propylene Diene 50 Shore A Grey RAL 7035...
- Page 9 PET (Polyester), Pantone Elastic band 1680 Blue Spandex 288C Title page of Instruction Manual PM-1840-01-02/2020...
- Page 10 Unfolded package design of main device and accessories Main device: Accessories: Nasal Shower NEB6014 PM-1840-01-02/2020...
- Page 11 List of Harmonized EN Standards The device is classified as a medical device, class IIa. Applicable Medical Device Directive (93/42/EEC) standards: EN 13544-1:2007+A1 :2009 EN ISO 15223-1:2016 EN 1041:2008+A1 :2013 UNI EN ISO10993-1:2010 EN 10993-5 :2009 EN 10993-10:2010 EN 60601-1:2006/A11 :2011/A1 :2013 EN 60601-1-2:2015 EN 60601-1-11:2015 EN 60601-1-6:2010+A1:2015...
-
Page 12: Maintenance
Make sure that the air tube is connected to the compressor. Disconnect the air tube from the nebulizer kit. Turn on the compressor and dry the air tube by passing air through it. Disinfecting the Nebulizer Kit, Mask, Mouthpiece and Adapter: Always disinfect the parts before using the product for the first time and after the product has not been used for an extended period of time as well as after the last treatment of the day. - Page 13 Storage Do not store the air tube while there is moisture remaining inside. •Never leave the nebulizer kit with medication near the ventilation slots, this is to avoid medication entering the device. • Store the parts in a clean location for avoiding infection. •...
- Page 14 9. For type, dose, and regime of medication follow the instructions of your doctor or respiratory therapist. 10. This product should not be used on patients, who are unconscious or are not breathing spontaneously. 11. After finishing the treatment remember to disconnect the air tube from the nebulizer kit and from the compressor.
- Page 15 Do not attempt to repair the device. Do not open and/or tamper with the device. During the period of warranty OMRON will, without charge for labour or parts, repair or replace the defect product or any defective parts. Repair or replacement under the warranty does not give rise to any extension or renewal of the warranty period.