Subscribe to Our Youtube Channel
Summary of Contents for Innovative Neutronics WalkAide
- Page 1 © 2010 Innovative Neurotronics. All rights reserved. All trademarks and registered trademarks are the property of their respective holders. LM01 R9...
- Page 2 System User Manual Caution: USA Federal Law restricts this device to sale by or on the order of a physician.
- Page 3 Medical Device & QA Services 76, Stockport Road 0086 Timperley, Cheshire WA15 7SN United Kingdom Tel: 44 161 870 6751 e-mail: info@mdqaservices.com...
-
Page 4: Table Of Contents
4.3 Skin Care 4.4 Instructions for Exercise Mode 4.5 Changing the Battery 4.6 Changing the Electrodes Maintenance and Cleaning of the WalkAide and Accessories Troubleshooting—Frequently Asked Questions Wearing Schedule WalkAide User Statement of Understanding Medical ID Card 10.0 Technical Information... -
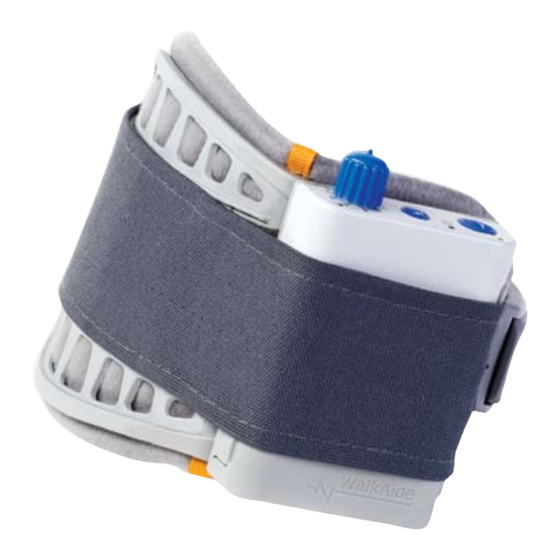
Page 5: The Walkaide Stimulator System
The WalkAide Patient Kit consists of a WalkAide Control Unit, a WalkAide cuff, and an electrode lead cable. A foot sensor is an optional item. -
Page 6: Indications Of Use
Indications of Use The Innovative Neurotronics WalkAide System is a surface functional neuromuscular stimulator system that is intended to address foot drop in patients who have sustained damage to upper motor neurons or pathways in the brain or spinal cord. During the swing phase of walking, the... -
Page 7: Warnings About Fes
EKG machines. However, the operation of the FES device will not be affected by the use of electronic monitoring equipment. MRI—The WalkAide should not be worn while receiving an MRI scan. Electrodes—The use of electrodes not supplied by Innovative Neurotronics may diminish results or increase risk of burns or discomfort. -
Page 8: Walkaide Specific Warnings
The WalkAide is not designed to prevent falling. Electrodes—The user should not relocate the position of the electrodes within the cuff. Do not use the WalkAide without electrodes. Placement—Never use the WalkAide on any area of the body other than the leg. -
Page 9: Precautions
Regularly check accessories for wear and replace as needed. Electrodes should be firmly secured to the skin. Never use the WalkAide if it appears to be malfunctioning. If there is a change in the way it usually works (i.e. change in sensation, surging of stimulation, intermittent stimulation) do not use the WalkAide and contact your clinician immediately. -
Page 10: Adverse Reactions
Signs of irritation are maintained redness, small pimple-like lesions or blisters. DO NOT continue stimulation over irritated skin. Notify the medical doctor if these conditions persist and discontinue use of the WalkAide until the problem is resolved. -
Page 11: Cautions
DO NOT use the WalkAide if the equipment is not operating properly. NEVER use the WalkAide unit with frayed or broken leads. ALWAYS handle the unit carefully. DO NOT expose the unit to water, excessive heat or vibration. -
Page 12: Symbols And Definitions
2.0 Symbols and Definitions Type BF Equipment Indicates Error Signal Indicates battery location and positioning Indicates impulse, STIM button Indicates connector location for optional Patient Foot Sensor Indicates input/output connector location for WalkLink Indicates exercise button Important safety instructions... -
Page 13: Walkaide Controls And Indicators
Connector for standard AA Output Connector [for clinician use only] (10) [if provided to user] (8) Alkaline battery (9) for Electrode Lead Cable (7) Back Left side Front Right side Figure 3: Back, side(s) and front views of WalkAide unit... -
Page 14: General Operating Instructions
The WalkAide is designed for single-handed application and removal (Figure 4). It may take a bit of practice to develop a routine that works best for each person. The WalkAide is applied directly to the leg and can be easily worn under most clothing. Applying... -
Page 15: Applying The Walkaide
Moisten the electrodes with water and place the cuff in the correct position below the knee. The electrodes will be on the outside of the leg and the WalkAide unit will be on the inside of the leg just below the knee. - Page 16 Always adjust the intensity level to the level determined by the clinician. Higher levels of stimulation may result in discomfort or skin irritation. Stand up and walk as usual. The WalkAide can be used with or without shoes, although proper footwear is recommended.
-
Page 17: Removing The Walkaide
Keep the resealable plastic bag and unit out of direct sunlight. The WalkAide may be worn all day but must be removed at night before going to bed. Be sure to turn the WalkAide unit OFF to prevent unintentional stimulation during handling and to conserve battery power when it is not being worn. -
Page 18: Instructions For Exercise Mode
Adjust the Intensity Knob (1) and then press the Exercise Button (3) for more than 3 seconds (Figure 7). An amber light (5) will flash on the top of the WalkAide unit and will beep. This will start the intermittent stimulation. -
Page 19: Changing The Battery
The WalkAide unit will stop stimulating when the programmed exercise session is finished. Turn the unit OFF. After 1–2 seconds, the WalkAide can be turned on again (automatically returning to walking mode) and the stimulation intensity can be adjusted to the desired level for walking. -
Page 20: Changing The Electrodes
1 to 2 weeks. Pull back the WalkAide attachment strap to uncover the electrode lead cables. Disconnect the black and red leads between he WalkAide and the electrodes. Remove the electrodes from the Electrode Locators. Place the new electrodes on the Electrode locators and feed the leads through the holes toward the outside of the cuff. -
Page 21: Maintenance And Cleaning Of The Walkaide And Accessories
DO NOT use a strong cleaning solution that contains bleach or immerse the WalkAide unit in water. Storing the WalkAide If the WalkAide is to be stored for an extended period of time and not used, remove the battery from the battery compartment. -
Page 22: Troubleshooting-Frequently Asked Questions
WalkAide and contact the clinician. 2. Why is the green light blinking while the red light is on? This is an error message. Turn the WalkAide off and wait 2-3 seconds. Turn the WalkAide back on and check to see that the green light is blinking and the red light is off. - Page 23 10. Under what circumstances should the clinician be contacted? Contact the clinician any time there is/are: additional questions or concerns about the WalkAide and its proper use; a change in the medical condition or walking pattern; any WalkAide accessory shows excessive wear and tear; the WalkAide does not function properly;...
-
Page 24: Wearing Schedule
1 hour Helpful Hints to Enhance the Break-In Period • Remove the WalkAide system every 2 hours to check skin integrity. • Slowly work into full-time wearing of the WalkAide system. • Remove the cuff at regular intervals and inspect the skin under the electrodes. -
Page 25: Walkaide User Statement Of Understanding
WalkAide System User Manual with my practitioner. I understand the general operating instructions and general maintenance of the WalkAide System. I have been advised to follow the wearing schedule and been advised to contact my practitioner immediately with any questions I may have with the WalkAide System. -
Page 26: Medical Id Card
9.0 Medical Device ID Card Medical Device ID Patient Name: Practitioner: Phone : Device: WalkAide System Innovative Neurotronics • Austin, TX 78746 • 1-888-884-6462 This card may be cut out and placed in your wallet for medical device identification purposes. -
Page 27: Technical Information
9.0 Medical Device ID Card (cont.) Innovative Neurotronics, Inc. 1.888.884.6462 www.ininc.us The Innovative Neurotronics WalkAide System is an external functional neuromuscular stimulator system. This FDA cleared medical device is battery operated and is worn below the knee. It is worn to assist patients diagnosed with foot drop in their walking ability. - Page 28 Expected Service Life: 5years Do not hesitate to contact your clinician should you have any questions regarding this device and its safe operation and use. If further assistance is required, please contact: Innovative Neurotronics, Inc. Austin, TX 888-884-6462 • www.walkaide.com...


Need help?
Do you have a question about the WalkAide and is the answer not in the manual?
Questions and answers