Summary of Contents for Roscoe Medical US PRO 2000 2nd Edition DU3035
- Page 1 Portable Ultrasound US PRO 2000 Edition ™ INSTRUCTION MANUAL Model # DU3035 CAUTION: Federal Law restricts this device to sale by or on the order of a physician or licensed practitioner...
-
Page 2: Declaration Of Conformity
Portable Ultrasound Unit This user manual is published by Roscoe Medical Inc. Roscoe Medical does not guarantee its contents and reserves the right to improve and amend it at any time without prior notice. Amendments will however be published in a new edition of this manual. -
Page 3: Table Of Contents
TABLE OF CONTENTS 1. FORWARD 2. INTENDED USE 3. EXPLANATION OF ULTRASONIC STIMULATOR EFFECT 4. CONTRAINDICATIONS 5. PRECAUTIONS 6. CAUTIONS 7. PARTS OF THE DEVICE 8. SPECIAL FEATURES 9. STEPS TO CONNECT THE ADAPTOR 10. INSTRUCTIONS FOR USE 11. LOAD DETECTION SYSTEM CAUTION 11. -
Page 4: Forward
FORWARD This manual contains general information on the operation, precautionary practices, and maintenance information of the DU3035 US Pro™ 2000 2nd Edition. In order to maximize the use, e ciency, and life of the device, please read the manual thoroughly and become familiar with it before operating the device. -
Page 5: Contraindications
CONTRAINDICATIONS 1. Do not use over or near bone growth centers until bone growth is complete. 2. Do not use over a healing fracture. 3. Do not use over the eyes. 4. Do not use on patients with implanted neurostimulation systems because tissue damage can occur at the location of the implanted electrodes resulting in severe injury or death. -
Page 6: Cautions
CAUTIONS 1. Always use this device under the directions of a physician. 2. Patients with the following diseases, symptoms or conditions should not use the device: During pregnancy or menstrual cycle. Acute disease, heart disease, tubercle disease, facial neuralgia (sharp facial pain), pernicious tumor, hemophilia, high fever, abnormal blood pressure, or under any unhealthy conditions. -
Page 7: Parts Of The Device
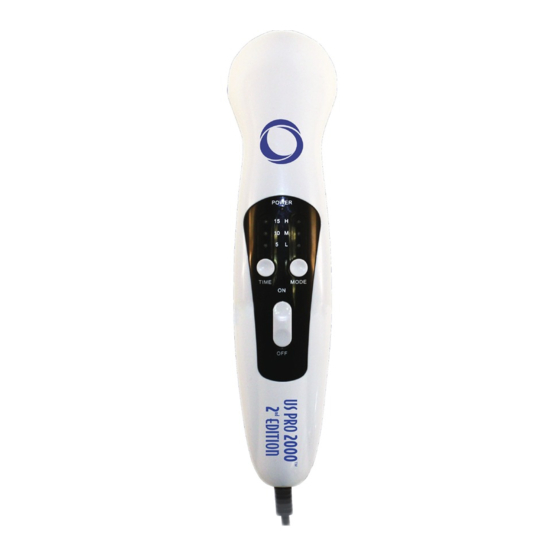
PARTS OF THE DEVICE (1) TIME INDICATOR LIGHT (2) TIME BUTTON (3) POWER INDICATOR LIGHT (4) INTENSITY INDICATOR LIGHT (5) MODE BUTTON (6) POWER SWITCH (7) ULTRASOUND HEAD SPECIAL FEATURES 1. All the ultrasound parts are assembled and tested under strict process controls. -
Page 8: Steps To Connect The Adaptor
US Pro™ 2000 2 Edition requires the following steps for proper setup: 1. Ultrasound transmission gel is required when treating a patient with the US Pro™ 2000 2nd Edition portable ultrasound device. 2. The AC/DC adapter is required to power the device. No battery is used. 3. -
Page 9: Instructions For Use
INSTRUCTIONS FOR USE Please read this instruction manual carefully before using the US Pro 2000 ™ Edition Portable Ultrasound Unit. 1. Turning on the device and head warming feature: Turn the device on by sliding the power switch upwards (towards “ON”). The power indicator light will illuminate. The device will automatically enter the preheat mode. - Page 10 3. Set ultrasound intensity: Press the “MODE” button to select the modulation duty cycle. The mode button has three levels, Low (L) - 5%, Medium (M) - 50% and High (H) - 100%, each level corresponds to a LED light indicator. 4.
-
Page 11: Load Detection System Caution
LOAD DETECTION SYSTEM CAUTION: 1. The device has a load detection system for safety. When the treatment head does not have good contact with the skin, the device will stop treatment automatically, and the time indicator light will flash one time. The device will not continue the treatment program until good contact is made. -
Page 12: Storage Conditions
STORAGE CONDITIONS When not in use, store the device with the adapter in a dry room and protect it against extreme moisture, heat and direct sunlight. Never place any heavy objects on the storage case. STORAGE CONDITIONS: 14°F ~ 122°F; 20% - 93% RH TROUBLESHOOTING The device is manufactured through complete quality assurance system. -
Page 13: Unit Specifications
UNIT SPECIFICATIONS Speci cations Item Description Ultrasound Modulation Frequency: 1.0MHz±10% Max. Output Power: 6.4W±20% (Modulation duty cycle at100%) Output Power: L: 0.32W±20% M 3.20W±20% H 6.40W±20% Pulse Repetition Rate: 100Hz±10% Modulation Duty Cycle: L (5%), M (50%), H (100%) E ective Radiating Area: 4.0cm²±... -
Page 14: Prescription Statement
PRESCRIPTION STATEMENT CAUTION: United States Federal Law restricts this device to sale by or on the order of a physician or licensed practitioner by the law of the State in which he/she practices, according to 21 CFR 801.109. GLOSSARY OF SYMBOLS Type BF Applied Part Caution Type of protection against electric shock: Class II Equipment... -
Page 15: Important Information Regarding Emc
Medical devices manufactured by Roscoe Medical, Inc. conform to this IEC60601-1-2:2007 standard for both immunity and emissions. Refer to EMC table guidance supplied in this manual regarding the EMC environment in which the device should be used. - Page 16 TABLE 1 Guidance and manufacturer’s declaration - electromagnetic emissions The DU3035 device is intended for use in the electromagnetic environment speci ed below. The customer or the user of the DU3035 should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment - guidance...
- Page 17 TABLE 2 Guidance and manufacturer’s declaration - electromagnetic immunity The DU3035 device is intended for use in the electromagnetic environment speci ed below. The customer or the user of the DU3035 should assure that it is used in such environment. IEC 60601 Compliance Electromagnetic...
- Page 18 TABLE 3 Guidance and manufacturer’s declaration - electromagnetic emissions The DU3035 device is intended for use in the electromagnetic environment speci ed below. The customer or the user of the Du3035 should assure that it is used in such an environment. IEC 60601 Compliance Electromagnetic...
- Page 19 TABLE 4 Recommended separation distances between portable and mobile RF communications equipment and the device The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and as recommended below, according to the maximum output power of the communications equipment.
-
Page 20: Limited Warranty
Product: US Pro™2000 2nd Edition Model: DU3035 Serial Number: Date of Purchase: Distributor: Manufactured for Roscoe Medical™, Inc. 21973 Commerce Parkway Strongsville, Ohio 44149 Ph: (800) 871-7858 www.roscoemedical.com...



Need help?
Do you have a question about the US PRO 2000 2nd Edition DU3035 and is the answer not in the manual?
Questions and answers
can the us pro 200 be used underwater?
can us pro 2000 be used underwater?