Advertisement
Quick Links
Instructions for Use
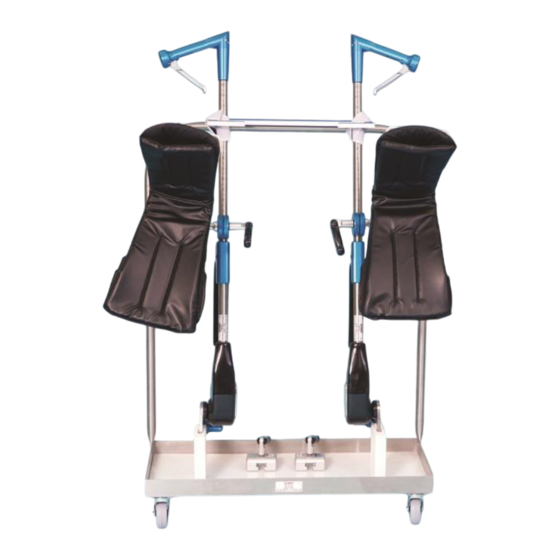
Robotic Stirrup Dolly 800-0074-R
INTENDED USE
Intended use is to store components of our Great White
Robotic Stirrups. The intended users of this device are
medical professionals within hospitals and surgery
centers.
INSTRUCTIONS
1. Position stirrup mounting blade as shown. If
mounting blade doesn't face down as shown, squeeze
trigger on handle and it will automatically position
itself.
2. Insert blade completely into blocks mounted on dolly
3. Locate left and right stirrup by label on bottom of
boot
4. Use hook and loop fastener straps provided on dolly to wrap around stirrups and fasten down
COMPONENT OVERVIEW
Robotic Stirrup Dolly is a storage cart used to store components of our Great White Robotic Stirrups.
GENERAL SPECIFICATIONS
Device Dimensions (maximum)
• Length: 27" +/- 0.5" (69 cm +/- 1 cm)
• Width: 12" +/- 0.5" (31 cm +/- 1 cm)
• Height: 38" +/- 0.5" (97 cm +/- 1 cm)
• Device Weight: 28 +/- 0.5 lbs. (12.7 +/- .22 kg)
• Fully Assembled
GENERAL INFORMATION
• Product not made with Natural Rubber Latex
• Product warranty covers product from manufacturing defects for period of 2 years
IFU-800-0074-R REV 3.05
Latest Revision: 2022-01
1
Advertisement

Summary of Contents for SchureMed 800-0074-R
- Page 1 Instructions for Use Robotic Stirrup Dolly 800-0074-R INTENDED USE Intended use is to store components of our Great White Robotic Stirrups. The intended users of this device are medical professionals within hospitals and surgery centers. INSTRUCTIONS 1. Position stirrup mounting blade as shown. If mounting blade doesn’t face down as shown, squeeze...
- Page 2 • General - Prevent infection by cleaning and disinfecting product before disposal • Packaging - Dispose packaging material via household waste according to national requirements • SchureMed accepts back used or retired products - or dispose of product in accordance with national requirements...
- Page 3 Indicates a barcode as containing unique device identifier information. CE Marking European Conformity. Single Patient Use Indicates the item is a single patient use medical device. Manufacturer SchureMed (081001460) Authorized Representative Emergo Europe, Prinsessegracht 20, 2514 AP The Hague, The Netherlands IFU-800-0074-R REV 3.05 Latest Revision: 2022-01...


Need help?
Do you have a question about the 800-0074-R and is the answer not in the manual?
Questions and answers