Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for inbody 580
- Page 1 User’s Manual...
- Page 2 1. Intended Use 2. Medical Indication The InBody is mainly used for healthy and acute or chronically • Medical check-up: Four body composition analysis can ill populations in hospitals, medical practices and inpatient be identified for the risk of developing diseases that are care facilities in accordance with national regulations.
- Page 3 3. Contraindication Individuals with medical implant devices such as pacemakers, or essential support devices such as patient monitoring systems, must not use this equipment. Safe, low-level currents will flow through the body during the test, which may cause malfunctioning of the device or endanger lives. Individuals with known metal allergies against stainless steel materials shall not use the equipment.
- Page 4 Please read before use and keep it in a safe place. Website: inbodyusa.com E-mail: info.us@inbody.com By following the manual instructions, you will be able to use the InBody more safely and effectively. InBody BWA Inc. [USA] 2550 Eisenhower Avenue, Suite C 209, Audubon, PA 19403 TEL: +1-610-348-7745 Headquarters Information Website: inbodybwa.com...
-
Page 5: Table Of Contents
8 Frequently Asked Questions (FAQ) .25 Installation ..........9 Regarding the Device ......25 4 Setting ..........11 Regarding of Serious Incidents ..25 Regarding the InBody Test ....25 Initial Setup ..........11 Setup .............11 Residual Risks and Undesirable Side Effects ...........26 Overview of the Setup Menu .....12 9 Classifications and Specifications ..27... -
Page 6: Safety
• When storing the device for a long period of time, store it endanger lives. InBody Co., Ltd. shall not be liable for any on a flat surface after turning off the device, unplugging damages to an individual or an equipment that occurred by the adapter, and packing the device. -
Page 7: Product Overview
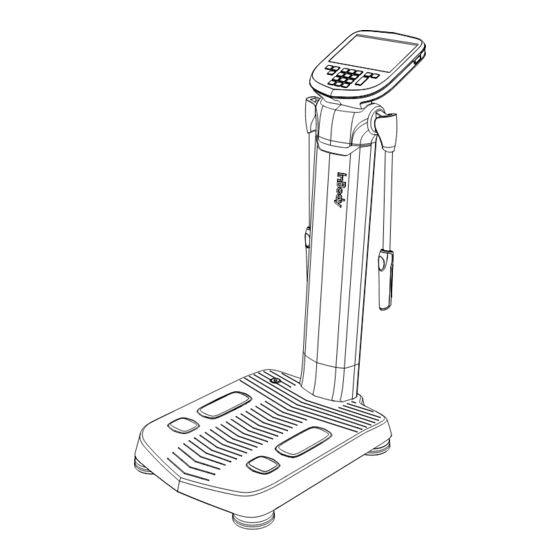
• The specifications of power cables can vary by country. Delete: Used to delete the saved data. Hand Electrode: Sends a weak current to the upper body and measures the voltage for the InBody test. 2.3 Optional Product Smart Reader: InBody App user can transfer the personal information needed for the test with QR code. - Page 8 Supporter: Can fit the level by adjusting height. Power Inlet: Connect a power adapter. Note • Make sure to connect only InBody stadiometer, Blood Pressure Monitor and SD400. • When connecting to a PC where the data management program LookinBody120 is installed, you can connect with one of the serial port, Bluetooth, LAN, or Wi-Fi port.
-
Page 9: Installation
3 Installation 3.1 Installation Environment Raise the upper part of the device and remove the protective packaging vinyl. Caution Protective Packaging Vinyl • If you are operating the device in a place where the altitude is 2,000m or higher, the weight measurement may be affected. - Page 10 Power Cable Power Cord Caution • Use the power adapter provided by InBody. The device may malfunction when using other adapters. • The device may get damaged or malfunctioned due to the electric if plugging into an ungrounded outlet. Or the test results may be inaccurate.
-
Page 11: Setting
Note You can change the initial settings at any time in Setup. 4.2 Setup When InBody device is booted for the first time, the initial setting screen appears. Complete the initial setup selecting the appropriate item. Press Start InBody after completing initial setting. -
Page 12: Overview Of The Setup Menu
Enter the administrator password and press Enter. • This screen will appear only once for initial password Menu setup. Set your InBody device fits to your facility and manage the data. General • Date and Time: Set the date and time displayed at the test standby screen and on the result sheet. - Page 13 Setting Connect Detailed Result Sheet Settings • InBody Result Sheet: You can set whether to use the • Internet: This option allows connecting the InBody device to the Internet. Once the device is connected to InBody Result Sheet and the Result Sheet Items (Body...
-
Page 14: Connecting Compatible Device
• When InBody device is already turned on, the stadiometer might not properly connect. Connect the serial cable supplied with the stadiometer to the right serial port on the rear of the InBody device. Connect the other end of the cable to the serial port of the stadiometer. -
Page 15: Barcode Reader
Connect the serial cable supplied with the SD400 to USB HOST port on the rear of the InBody. the left serial port on the rear of the InBody device. Connect the other end of the cable to the serial port of the SD400. - Page 16 InBody Customer Service. Note • Refer to the User’s manual of LookinBody120 for more details on how to setup Bluetooth on LookinBody120. • You can check the Bluetooth ID of InBody in Setup > 3.Connect > Bluetooth.
-
Page 17: Connecting Internet
Connecting Wi-Fi Press Setup on the test standby screen or Setup Once InBody device is connected to the Internet, you can button on the keypad of the operation panel before use it to connect the Cloud Services or LookinBody120. stepping on the footplate. -
Page 18: Optional Device's Specification
• Memory (RAM): 1GB or higher recommended • Graphics cards and monitors: Resolution 1024x768 5.10 IT security measures Interfaces and network protocol InBody would like to clarify that the user access to the Interfaces Protocol InBody580 is only granted for the authorized users, who have appropriately registered the passcode in the system IEEE 802.11 b/g/n 2.4GHz... -
Page 19: Inbody Test
Stand onto the footplate with bare feet on the standby endanger lives. InBody Co., Ltd. shall not be liable for any screen. damages to an individual or a device that occurred by not •... - Page 20 InBody Test Input the InBody User ID or height and press Next. Maintain test posture. • Professional Mode: Input ID. • For proper test posture, refer to “6.3 Test Posture”. • Self Mode: Input height, age, gender. • The InBody test automatically starts when the body and the electrode are in precise contact.
- Page 21 InBody Test The result screen is displayed after completing the test. • The Result Sheet is printed out according to your setting when the printer is connected. • Please refer to “4.3 Overview of the Setup Menu” “5.1 Printer” for details of printer and Result Sheet setting.
-
Page 22: Test Posture
InBody Test 6.3 Test Posture The subject must maintain proper posture to have accurate test results. • The InBody test automatically starts when the body and the electrode are in precise contact. Your arms must not touch the sides of your body. -
Page 23: Maintenance And Storage
When repacking the device, the protective packaging • Be careful not to get foreign materials on the bottom of materials provided by InBody must be used. the device. It may cause weight measurement errors. • Be careful not to get injured by getting your feet caught in the bottom of the device. -
Page 24: Storage Environment
Maintenance and Storage 7.5 Storage Environment The device should be stored under the following conditions. ‒10 - 70 ℃ (14 ~ 158°F) Temperature range Relative humidity 10 - 80 % RH (No Condensation) Atmospheric pressure 50 - 106 kPa... -
Page 25: Frequently Asked Questions (Faq)
8.1 Regarding the Device 8.2 Regarding of Serious Incidents Question: InBody Result is not sent to the App. Answer: If you are aware of a serious incident involving your product, • Check whether the Internet connection or communicate a corrective action to you clients, you must... -
Page 26: Residual Risks And Undesirable Side Effects
Question: I have limited mobility and cannot maintain proper posture for the InBody Test. How can I still be tested? • The test is available, but the test result may Answer: be inaccurate due to poor contacting to the electrode surface. -
Page 27: Classifications And Specifications
9 Classifications and Specifications • This device was produced according to InBody’s quality • Body Composition Analysis (Total Body Outputs control procedures. InBody complies with ISO9001 and Water, Protein, Minerals, Body Fat Mass, (InBody Result ISO13485, international quality management systems. - Page 28 • Obesity Analysis (Body Mass Index, LookinBody120 Percent Body Fat) • Growth Curve Outputs (Height, Weight, Types of InBody Result Sheet, InBody Result Sheet BMI) for Children Result Sheets • Body Composition History (Height, Weight, BMI, Skeletal Muscle Mass, Soft...
-
Page 29: Symbols Used On The Product
Classifcations and Specifcations 9.3 Symbols used on the Other specifications Product Applied Rating 200 μA (±20 μA) Current Indicators Power Input AC 100-240 V, Adapter ① 50/60 Hz, 1.2 A (Bridge Power) Power Output DC 12 V, 3.4 A 9-pin Serial Port (Female, RS-232C) Power Input AC 100-240 V, Adapter ②... - Page 30 Classifiaaiiossaons Spiifiaaiios Operating instructions Catalogue number Importer Country of manufacture Disposal of old Electrical & Electronic Equipment (Application in the European Union and other European countries with separate collection system.) This symbol indicates that this product shall not be treated as household waste. Instead, it shall be handed over to the applicable collection point for the recycling of electrical and electronic equipment.
-
Page 31: Guidance And Manufacturer's Declaration
Classifcations and Specifcations 9.4 Guidance and Manufacturer’s Declaration The InBody device is intended for use in the electromagnetic environment specified below. The customer or the user of the InBody device should ensure that it is used in such an environment. - Page 32 The InBody device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the InBody device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the InBody device as recommended below, according to the maximum output power of the communications equipment.
- Page 33 If the measured field strength in the location in which the InBody device is used exceeds the applicable RF compliance level above,the InBody device should be observed to verify normal operation. If abnormal performance is observed,additional measures maybe necessary, such as re-orienting or relocating the InBody device.
- Page 34 The InBody device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. Portable RF communications equipment should be used no closer than 30 cm (12 inches) to any part of the InBody device. Otherwise, the performance of this equipment could be impaired.
-
Page 35: Key Performance Claims Of Inbody580
Analyzer of InBody (Models: InBody580) provides clinical d'actionner le dispositif de déconnexion. benefits to support the aforementioned intended use, as the of InBody (Models: InBody580) in mainly used for healthy and acute or chronically ill populations in hospitals, medical practices and inpatient care facilities in accordance with national regulations. - Page 36 © 2024 InBody Co., Ltd. All rights reserved. IM-ENG-R6-B-240111...


Need help?
Do you have a question about the 580 and is the answer not in the manual?
Questions and answers