Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Richmar InTENSity 10
- Page 1 INTENSITY™ 10 INSTRUCTION MANUAL INTENSITY INTENSITY...
- Page 2 This manual is valid for the InTENSity™ 10 TENS Stimulator This user manual is published by Compass Health Brands. Compass Health Brands does not guarantee its contents and reserves the right to improve and amend it at any time without prior notice. Amendments may however be published in new editions of this manual.
-
Page 3: Table Of Contents
TABLE OF CONTENTS 1. GENERAL INFORMATION _______________________________ 4 1.1 Device Information 1.2 Medical Background 1.3 Indication for Use 2. IMPORTANT SAFETY INFORMATION ______________________ 5 2.1 Contraindications 2.2 Warnings, Cautions, Adverse Reactions 3. PRESENTATION _______________________________________ 10 3.1 Front and Rear Panel 3.2 LCD Display 4. -
Page 4: General Information
1. GENERAL INFORMATION 1.1 Device Information InTENSity™ 10 TENS stimulator is a portable electro-therapy device featuring Transcutaneous Electrical Nerve Stimulation (TENS), which is used for pain relief and electrical muscle stimulation. The stimulator sends a gentle electrical current to underlying nerves and muscle group via electrodes applied on the skin. -
Page 5: Indication For Use
HOW TENS WORKS There is nothing "magic" about Transcutaneous Electrical Nerve Stimulations (TENS). TENS is intended to be used to relieve pain. The TENS unit sends comfortable impulses through the skin that stimulate the nerve (or nerves) in the treatment area. In many cases, this stimulation will greatly reduce or eliminate the pain sensation the patient feels. -
Page 6: Contraindications
2.1 Contraindications This device should not be used for symptomatic local pain relief unless etiology is established or unless a pain syndrome has been diagnosed. 2. This device should not be used when cancerous lesions are present in the treatment area. 3. - Page 7 7. Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known sensitivity to the carotid sinus reflex. 8. Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal muscles may occur and the contraction may be strong enough to close the airway or cause difficulty in breathing.
- Page 8 CAUTIONS: Federal law (USA) restricts this device to sale by or on the order of a physician. 2. This device is for single patient use only. 3. Keep yourself informed of the contraindications. 4. This device is not intended for use on an unattended patient who is non-compliant, emotionally disturbed, has dementia, or low IQ.
- Page 9 17. The electrodes are only to be placed on healthy skin. Avoid skin irritation by ensuring that good contact is achieved between electrodes and skin. 18. If the stimulation levels are uncomfortable or become uncomfortable, reduce the stimulation intensity to a comfortable level and contact your physician if problems persist.
-
Page 10: Presentation
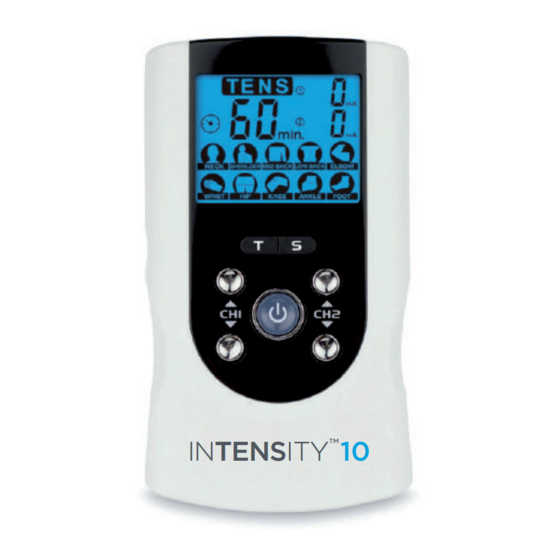
3. PRESENTATION 3.1 Front and Rear Panel INTENSITY INTENSITY Output socket: electric signal output of the cable on channel 1 & 2. 2. AC Adaptor connecting port. 3. T(time) and S(therapeutic part) setting buttons. 4. Increases [▲] the output intensity of channel 1. 5. -
Page 11: Lcd Display
3.2 LCD Display Displays therapeutic mode (TENS). 2. Lock function indicator. 3. Low-battery indicator. 4. Displays the cycle time of the treatment. 5. Timer symbol. 6. Displays the numerical value of the output intensity for channel 1. 7. Displays the numerical value of the output intensity for channel 2. 8. -
Page 12: Specification
4. SPECIFICATIONS 4.1 Accessories DESCRIPTION TENS Stimulator Device (Item: DI1010) 1 each Pair of Lead wires (Item: WW3005) 2/pk 2” x 2” Self Adhesive electrodes 4/pk (Item: EP2020WC2-INTM) 9V TENScell alkaline battery 1 each (Item: TA5013-I) Instruction manual 1 each Carrying case (Item: CC5082) 1 each Wall (AC) adapter (Item: DI0001X) -
Page 13: The Waveforms Of The Stimulation Programs
4.3 The Waveforms of the Stimulation Programs Normal Pulse Width Modulation Pulse Rate Modulation Modulation (Pulse Width and Pulse Rate) -
Page 14: Instructions For Use
5. INSTRUCTIONS FOR USE 5.1 Battery 5.1.1 Check/Replace the Battery Over time, in order to ensure the functional safety of the device, changing the battery is necessary. Slide the battery compartment cover to open. 2. Insert the 9V battery into the battery compartment. -
Page 15: Connect Electrodes To Lead Wires
CAUTION FOR BATTERIES: Swallowing a battery may be fatal. Keep the battery and the device out of the reach of children. If a battery is swallowed, consult a physician immediately. 2. If a battery has leaked, avoid contact with skin, eyes and mucus membranes. -
Page 16: Connect Lead Wires To Device
5.3 Connect Lead Wires to Device Before proceeding to this step, be sure the device is completely turned OFF. 2. Insert the wires provided with the system into the jack sockets located on top of the device. 3. Holding the insulated portion of the connector, push the plug end of the wire into one of the jacks (see drawing);... - Page 17 5.4.1 Place Electrode on Skin Apply electrodes to the exact site indicated by your physician or therapist. Before applying electrodes, be sure the skin surface over which electrodes are placed is thoroughly cleaned and dry. Make sure the electrodes are pressed firmly to the skin and make good contact between the skin and the electrodes.
-
Page 18: Select The Therapeutic Program
5.5 Turning On the Device Before using the device for the first time, you are strongly advised to take careful note of the contraindications and safety measures detailed at the beginning of this manual (pages 5-9 and throughout manual), as this powerful equipment is neither a toy nor a gadget! To turn on the device, PRESS and RELEASE the ON/OFF button. -
Page 19: Safety Lock Feature
2. When using the device in active mode, if the electrodes are not placed firmly on the skin or the device is not connected to the electrodes and the stimulator’s output intensity surpasses 12mA, the intensity will automatically reset to 0mA. 5.9. -
Page 20: Program
6. PROGRAM Therapeutic Waveform Frequency Pulse Wide Treat. time Part Program (Hz) ( μ (Min) default Neck Modulation 60-100 100-150 Shoulder Pulse Rate 80-100 modulation Mid Back Pulse Rate 100-150 modulation Low Back Frequency 50-80 modulation Elbow Continuous Wrist Continuous Pulse Rate 100-150 modulation... -
Page 21: Cleaning And Care
7. CLEANING AND CARE 7.1 Tips for Skin Care Follow these suggestions to avoid skin irritation, especially if you have sensitive skin: Wash the area of skin you will be placing the electrodes on with soap. Rinse thoroughly and dry the area completely before and after placing electrodes. - Page 22 TO REMOVE YOUR ELECTRODES: Lift the corner of the electrode and gently remove it from the skin. 2. It may be helpful to improve repeated electrode application by spreading a few drops of cold water over the adhesive side and turn the surface up to air dry.
-
Page 23: Cleaning The Electrodes Cords
7.4 Cleaning the Electrode Cords Clean the electrode cords by wiping them with a damp cloth. Coating them lightly with talcum powder will reduce tangles and prolong their life. 7.5 Maintenance Maintenance and all repairs should only be carried out by an authorized agency. -
Page 24: Troubleshooting
8. TROUBLESHOOTING If your device does not seem to be operating correctly, refer to the chart below to determine what may be wrong. Should none of these measures correct the problem, the device should be serviced. Problem Possible Cause Solution Display fails to Battery contact failure. - Page 25 Stimulation is Improper electrode and Reposition electrode and applicator ineffective applicator placement unknown Contact clinician The skin Using the electrodes on the Reposition the electrodes. If at any becomes red same site every time time you feel pain or discomfort and/or you feel stop use immediately.
-
Page 26: Storage
9. STORAGE For prolonged pauses in treatment, store the device in a cool dry room and protect it against heat, sunshine and moisture and remove the battery to avoid battery leaking. 2. Store the device in a cool, well-ventilated place. 3. -
Page 27: Electromagnetic Compatibility (Emc) Tables
11. ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES Use of accessories, transducers and cables other than those specified or provided by the manufacturer of this equipment could result in increased electromagnetic emissions or immunity of this equipment and result in improper operation. Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. - Page 28 Guidance and manufacturer’s declaration - electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance Electromagnetic level...
- Page 29 Immunity IEC 60501 Compliance Electromagnetic environment guidance test test level level Portable and mobile RF Communications equipment should be used no closer to any part of the device, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
- Page 30 Recommended separation distances between portable and mobile RF communications equipment and the device. The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) as recommended below, according to the maximum output power of the communications equipment...
-
Page 31: Glossary Of Symbols
12. GLOSSARY OF SYMBOLS Batch code Serial number Attention: Read the operating instruction before use! Equipment capable of delivering output values in excess of 10mA r.m.s. or 10V r.m.s. averaged over any period of 5s. Electrical devices are recyclable material and should not be disposed of with household waste after their useful life! Help us to protect the environment and save resources and take this device to the ap- propriate collection points. -
Page 32: Warranty
13. WARRANTY Please contact your dealer in case of a claim under the warranty. If you have to send the unit back to your provider, enclose a copy of your receipt and state what the defect is. The following warranty terms apply: The warranty period for device is one year from date of purchase (accessories, minus electrodes, have a six month warranty). - Page 33 4. Returned merchandise must be in the same unit of measure as originally purchased. 5. Return Labels or Call Tags can be issued by our customer service department to return merchandise. 6. Associated fees and return freight charges will apply. All returns of dropshipped items are subject to a restocking fee as well as inbound and outbound freight charges.
- Page 36 Manufactured for: 3284.19.06.C ©2023 Compass Health Brands Corp.



Need help?
Do you have a question about the InTENSity 10 and is the answer not in the manual?
Questions and answers