Summary of Contents for ActivePure Medical Guardian
- Page 1 MODEL: F171A (230V) READ MANUAL CAREFULLY FOR PROPER USE AND OPTIMAL OPERATION. ActivePure Medical ™ 14841 Dallas Parkway, Suite 500 | Dallas, TX 75254 Healthcare Inspired, Science Powered.
-
Page 2: Important Safety & Use Instructions
• Read the maintenance instructions before opening the front door • DO NOT attempt to repair or adjust any electrical or mechanical functions of the ActivePure Medical Guardian. Contact Customer Service for service support at 1.800.572.6241 • This device will automatically turn off when the door is open •... -
Page 3: Table Of Contents
BEFORE YOU BEGIN USING THE ACTIVEPURE MEDICAL GUARDIAN First, read this user manual in its entirety to ensure that the ActivePure Medical Guardian device is used as intended. Then plug it in, turn it on to the appropriate fan setting. The ActivePure Medical Guardian is designed for quiet operation, as not to disturb people in a professional healthcare environment. -
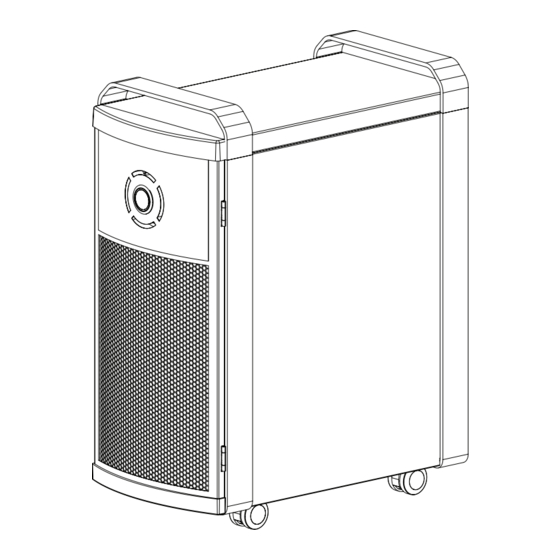
Page 4: Device Description
10-watt UVGI bulbs operating at a wavelength of 254nm; two pieces of polycarbonate honeycomb material with a combined surface area of 708 square inches, coated with a TiO2 based catalyst called ActivePure; power switch; circuit board which controls the device operations and an internal fan to draw air through the device, as shown in the general device design (Figure 1). -
Page 5: Product Features
PRODUCT FEATURES It is important to be familiar with the components of the ActivePure Medical Guardian. If any components are missing, contact Customer Service for support at 1.800.572.6241 or email at info@ActivePureMedical.com. Door Pull Back Handle Panel Airflow Input Front Panel... -
Page 6: Device Specifications
(673mm H x 292mm W x 533mm D) OPERATION Place the ActivePure Medical Guardian in a location that allows air to move freely into, around, and out of the unit. Allow 12" of clearance (minimum) around the intake grille to ensure adequate airflow. -
Page 7: Treatment Space
TREATMENT SPACE The ActivePure Medical Guardian, model F171A is a device intended for medical purposes that is used in professional healthcare environments. The device is designed to treat a space approximately 3,000 cubic feet that would have 8’ to 10’ ceilings. Recommended mode of action is to operate the device on the highest speed possible continuously. -
Page 8: Settings
365 days (1 year) of use. The ActivePure Medical Guardian has a safety shutdown feature to ensure the filter and/or cell replacement is performed at the appropriate times. When the filter and/or cell replacement time is reached, the appropriate LED indicator will illuminate. -
Page 9: Cleaning/Disinfecting And Filter Replacement Instructions
CLEANING/DISINFECTING AND FILTER REPLACEMENT INSTRUCTIONS The ActivePure Medical Guardian, model F171A is a device intended for medical purposes that is used for the reduction of staphylococcus epidermidis and erwinia herbicola bacteria, MS2 and Phi-X174 viruses and aspergillum niger fungal spores and bacillus globigii bacterial spores from the air in a temperature-controlled professional healthcare environment of 70~71°F, 40~45%RH. - Page 10 CLEANING/DISINFECTING AND FILTER REPLACEMENT INSTRUCTIONS (CONT.) Step 10: Remove a Metrex CaviWipe from its package. Begin by cleaning the surface “C”. Wipe the CaviWipe over the surface area, thoroughly wetting the soil. Then using hand pressure rub the wipe over the surface until soil is no longer visible on this part of the device.
-
Page 11: Activepure Cell Replacement Instructions
ACTIVEPURE CELL REPLACEMENT INSTRUCTIONS The ActivePure Medical Guardian, model F171A is a device intended for medical purposes that is used for the reduction of staphylococcus epidermidis and erwinia herbicola bacteria, MS2 and Phi-X174 viruses and aspergillum niger fungal spores and bacillus globigii bacterial spores from the air, in a temperature-controlled professional healthcare environment of 70~71°F, 40~45% RH. -
Page 12: Replacement Parts
10 seconds After you replace the filter or ActivePure Cell you will want to reset the indicator light(s). To do so simply press and hold the power button for 10 seconds. This will reset the filter and/or cell module replacement indicators. Only the illuminated indicator(s) will be reset when pressing the power button for 10 seconds. -
Page 13: Warranty Information
WARRANTY INFORMATION Limited Three (3) Year Warranty: To register your ActivePure Medical Guardian unit, call Customer Service at 1.800.572.6241 or email at info@ActivePureMedical.com. WHAT IS COVERED BY THIS WARRANTY LIMITATION OF LIABILITY FOR SPECIAL, INCIDENTAL OR ActivePure Medical, LLC warrants the ActivePure Medical Guardian,... -
Page 14: Device Iec 60601-1-2:2014 Emc Information
DEVICE IEC 60601-1-2:2014 EMC INFORMATION This device passed each of the 11 tests, as indicated. Immunity test levels performed on the device, • Electrostatic Discharge Immunity – Tested at 2,4,8 and 15 kV • Radiated Immunity – Tested 3 V/m (Professional Healthcare) and 10 V/m (Home Healthcare) •... - Page 16 For information regarding the use of this product please call Customer Service. 1.800.572.6241 or Email at info@ActivePureMedical.com Activepure.com ActivePure Medical, LLC | 14841 Dallas Parkway, Suite 500 | Dallas, TX 75254 | 1.800.572.6241 © ActivePure Medical, LLC. All Rights Reserved APMG_OM_65-00886_VA-01963...


Need help?
Do you have a question about the Medical Guardian and is the answer not in the manual?
Questions and answers