Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Medisoft FeNO+
- Page 1 HARDWARE USER MANUAL FeNO+ Version 2.2 H11-EN 30/12/2019...
-
Page 2: Table Of Contents
Content Foreword ........................4 Foreword ........................4 Description of available tests ..................5 List of standard and optional tests ................5 Intended users ......................6 Environmental conditions.................... 6 Warnings ........................7 Location of the device ....................7 General overview ...................... 8 Front panel........................ - Page 3 Revision Date Version Modifications 21/02/2016 1.0 Creation 01/06/2018 2.0 New design 14/05/2019 2.1 Modification of the CE marking 30/12/2019 2.2 Removing redundant information’s H11-EN FeNO+ 3/31...
-
Page 4: Foreword
The FeNO+ is a device with both exhaled NO analysis and spirometry functions dedicated for prediagnosis and monitoring of inflammatory disease such as asthma. The FeNO+ is operated by Expair, a software program developed by Medisoft S.A. that functions on Windows based PC systems. -
Page 5: Description Of Available Tests
1.2 Description of available tests The FeNO+ device allows two types of measurement: • Measurement of endogenous nitric oxide • Conventional spirometry (optional) NO endogenous measurement The measurement of NO endogenous includes: • Bronchial NO measurement at 50 ml/s exhalation flow rate (standard measurement) •... -
Page 6: Intended Users
1.4 Intended users This device is to be used by physiologists, doctors, respiratory therapists or nurses, or under supervision of such. Data obtained must be interpreted and reported by trained medical staff only. 1.5 Environmental conditions This device is for clinical use in hospitals, private doctor’s offices, medical schools, sports medicine facilities or universities. -
Page 7: Warnings
Warnings 2.1 Location of the device Location of the FeNO+ The FeNO+ module should not be located on wet or dusty conditions. The cooling ventilator on the rear panel must not be obstructed which could lead to an overheating inside the module. H11-EN Warnings 7/31... -
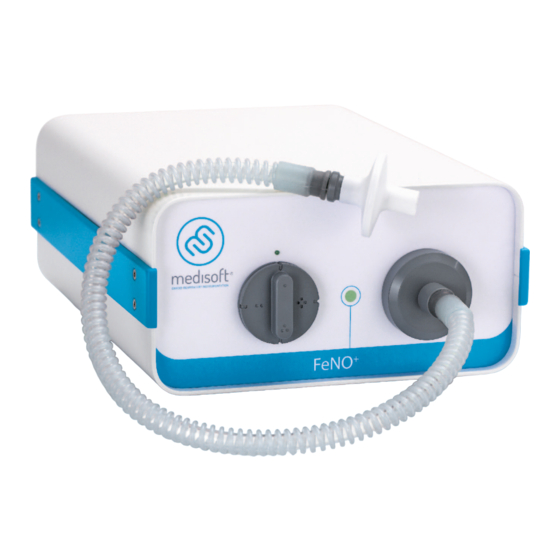
Page 8: General Overview
General overview 3.1 Front panel 3.1.1 Basic version Front panel Power LED Main sampling port Flow selector Corrugated tube for exhaled air sampling Antibacterial/viral filter H11-EN General overview 8/31... -
Page 9: Spirometry Option
3.1.2 Spirometry option Front panel Power LED Main sampling port Flow selector Corrugated tube for exhaled air sampling Antibacterial/viral filter Handheld spirometer Lilly type pneumotachograph Combined tubing for handheld spirometer Downstream flow connector (-,blue) Upstream flow connector (+,red) Heated wire connector Holder for pneumotachograph H11-EN General overview... -
Page 10: Rear Panel
3.2 Rear panel Rear panel Electrochemical NO sensor Ambient NO scrubber USB port (connected to computer) Gas sampling inlet Cooling fan Calibration gas bottle inlet Power switch (ON/OFF) Power supply entry H11-EN General overview 10/31... -
Page 11: Handheld Pneumotachograph
3.3 Handheld pneumotachograph Available with spirometry option Front part (patient facing) Heating wire and plug Mesh Handle Back part (to ambient) Antibacterial/viral filter Upstream flow line and connector (+,red) Downstream flow line and connector (-, blue) H11-EN General overview 11/31... -
Page 12: Connections
Connections 4.1 Connections on the front panel 4.1.1 All versions Connect the corrugated tube with antibacterial/viral filter adapter to the main inlet H11-EN Connections 12/31... -
Page 13: Spirometry Option
4.1.2 Spirometry option Insert the plug-in connectors to the upstream (red)/downstream (blue) ports Secure the connection by clockwise rotation of the plug-in connectors Plug the heated wire H11-EN Connections 13/31... -
Page 14: Connections On The Rear Panel
4.2 Connections on the rear panel Power supply cable USB cable Nasal catheter (for nasal option) H11-EN Connections 14/31... - Page 15 If the device has been turned off for more than days, the FeNO+ module needs a warm up time of 24 to 48 hours in order to polarize the electrochemical NO cell. Important information on NO electrochemical sensor polarization The FeNO+ module contains an electrochemical cell for Nitric Oxide measurement.
-
Page 16: Calibration
Calibration of the volume/flow measurement (Lilly pneumotachograph) NO gas Only performed by a qualified technician approved 6 months by Medisoft SA or its representative Calibration of the electrochemical NO sensor The module should be turned on at least 20 minutes before performing the calibration. -
Page 17: Initializing The Calibration Mode
5.3 Initializing the calibration mode On main menu of Expair, select the calibration menu Click on New Check ambient conditions and correct if necessary Define operator’s name H11-EN Calibration 17/31... - Page 18 Only if spirometry option is available The ambient conditions are used to calculate BTPS correction factor. It is essential that ambient conditions are measured or encoded correctly for accurate volume measurement: • Temperature: in degrees Celcius • Relative humidity: in % •...
-
Page 19: Volume Calibration
5.4 Volume calibration 5.4.1 Calibrating the pneumotachograph Calibration process H11-EN Calibration 19/31... - Page 20 Select the Volume tab. Select the device (if there are several modules connected to the same computer). Define the calibration syringe volume (by default, it is set to 3 liters). Click on Calibrate. Wait for the zero measurement (red cross). When the green check mark is displayed, the device is ready to calibrate.
- Page 21 Analysis of the volume calibration results Analysis of the calibration results is essential to ensure an accurate volume measurement by the system. Flow-volume loop During the calibration process, the flow-volume loops are displayed, the volume is on the horizontal axis and the flow is on the vertical axis. There should be no drift on the horizontal axis as shown below.
- Page 22 Verification of the system If one or two traffic lights are yellow or red, the operator has to perform a verification of the system described below. 1. Verify the connection of the tubing to the module (red and blue connectors). 2.
- Page 23 3. Verify the pneumotachograph is assembled correctly and fitted securely to the handle. 4. Verify the correct syringe volume (indicated on a label on the syringe) is the same volume entered into the software. 5. Verify the calibration syringe is not damaged and has no leak. Calibration syringes should ideally be checked and calibrated annually by a calibration laboratory.
- Page 24 Deviation between 5 and 10%. The calibration is acceptable, and the system can be used. The deviation could be a sign that device maintenance is necessary. Please contact your Medisoft representative. Deviation greater than 10%. The calibration is not acceptable. A verification of the system by a field service technician is required.
-
Page 25: Checking The Pneumotachograph Calibration
5.4.2 Checking the pneumotachograph calibration As the respiratory flow patterns are made up of many different flows, an individual flow error will affect the overall result. The calibration can be verified at multiple flow rates with the use of the Check button. This allows the operator to check different flow rates and to ensure the quality of the volume measurement linearity or that the readings are good over the full operating range of the pneumotachograph. - Page 26 ATS-ERS criteria. The system can be used. Accuracy greater than 3.5%. The linearity of the volume measurement is not good and does not meet ATS-ERS criteria. A verification of the system linearity by a field service technician is required. Please contact your Medisoft representative. H11-EN Calibration...
-
Page 27: Calibration
5.5 NO calibration The NO gas calibration has to be performed 1 x 6 months by a qualified technician approved by Medisoft SA or its representative. The procedure is only available in the FeNO+ service manual. The calibration requires a specific gas containing 100 ppb NO, balance N Two modes of calibration are available: •... -
Page 28: Maintenance
Maintenance 6.1 Foreword All Medisoft devices are designed to have a lifetime of 10 years minimum, which entails a regular maintenance to be performed by the manufacturer or its representative. The device requires a preventive maintenance once a year, along with a recommended bi-annual control visits in order to validate the calibration. -
Page 29: Maintenance Planning
6.2 Maintenance planning Table below shows the list of actions to be performed for a full preventive maintenance. Each action has to be performed by the user or a technician approved by Medisoft or its representative. The highlighted actions have to be performed by the user by following the procedures described in the next paragraphs. -
Page 30: Maintenance Procedures For The User
6.3 Maintenance procedures for the user 6.3.1 Cleaning See dedicated manual for cleaning and disinfection. 6.3.2 Replacement of the pneumotachograph mesh For devices that have the spirometry option. Remove the pneumotachograph from the handle. Take the pneumotachograph from the lower part and turn counter clockwise the upper part in order to disassemble the pneumotachograph. - Page 31 Medisoft S.A. www.medisoft.be P.A.E. de Sorinnes info@medisoft.be 1, Route de la Voie Cuivrée T: +32 (0)82 22 30 20 5503 Dinant – Sorinnes (Belgium) F: +32 (0)82 22 33 34...



Need help?
Do you have a question about the FeNO+ and is the answer not in the manual?
Questions and answers