Table of Contents
Advertisement
Quick Links
™
Synchrony
Equipment User Manual
Revised 10.01.2015
Part No. 290SYN Rev 1
This manual contains confidential and proprietary information owned by Accelerated Care Plus ("ACP"), division of Hanger Inc. which is
protected by copyright. This manual or any portion thereof may not be photocopied, reproduced or translated to another language without the
express prior written consent of ACP. This manual may only be used by entities who purchased the equipment or have implemented the ACP
program and are covered by an executed Lease Agreement with ACPL containing a Confidentiality Agreement, which is incorporated by
reference in its entirety. This manual may not be used for any other purpose.
Any additional copies of the manual shall be ordered from ACP. No changes or modifications shall be made to the manual without prior review
and written authorization from ACP. No authorization is given to market, sell, disclose, or exploit this manual except as for purposes of using the
equipment as contemplated by the Lease Agreement.
ACCELERATED CARE PLUS MAKES NO WARRANTY OF ANY KIND WITH REGARD TO THIS MANUAL, INCLUDING, BUT NOT
LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE.
Accelerated Care Plus and Hanger Inc., shall not be liable for errors contained herein or for incidental or consequential damages in connection
with the furnishing, performance or use of this manual. The information contained in this document is subject to change without notice.
© COPYRIGHT 2015, ACCELERATED CARE PLUS CORP., ALL RIGHTS RESERVED
CONFIDENTIAL AND PROPRIETARY
Advertisement
Table of Contents

Summary of Contents for Hanger ACP Synchrony
- Page 1 LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. Accelerated Care Plus and Hanger Inc., shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance or use of this manual. The information contained in this document is subject to change without notice.
- Page 2 Synchrony ™ ACP manufactures a premier line of rehabilitation technologies to assist health care professionals with improved outcomes and quality-of-life for patients. The ACP product line includes Pain Control Systems, Muscle Stimulators, Interferential Therapy, Therapeutic Ultrasound, Pulsed Shortwave Diathermy devices, and advanced Therapeutic ®...
-
Page 3: Table Of Contents
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL TABLE OF CONTENTS INDICATIONS & CONTRAINDICATIONS ....................- 1 - Indications for Use ........................... - 1 - Contraindications ............................. - 1 - WARNINGS & PRECAUTIONS ......................- 2 - Warnings ..............................- 2 - ... - Page 4 © COPYRIGHT 2015, ACCELERATED CARE PLUS CORP., ALL RIGHTS RESERVED CONFIDENTIAL AND PROPRIETARY...
-
Page 5: Indications & Contraindications
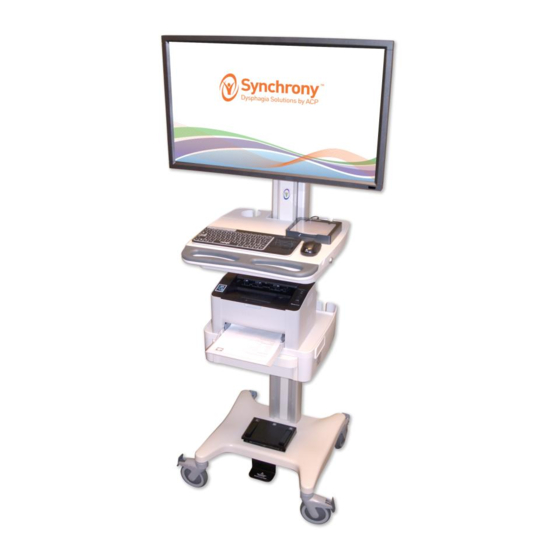
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 1 - INDICATIONS & CONTRAINDICATIONS Indications for Use ® Synchrony™ consists of a Dysphagia Cart, Omnistim Portable, and an OMNIsEMG™ surface electromyography (sEMG) biofeedback system. These devices are used in adjunct to oral motor and swallowing exercise and treatment. -
Page 6: Warnings & Precautions
- 2 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL WARNINGS & PRECAUTIONS Warnings Use the product according to its indications. Avoid connecting the sensors to the charger with inverted polarity, this may damage the sensors. The correct polarity is shown on the charger station. ... -
Page 7: Synchrony™ And Omnisemg™ Introduction
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 3 - SYNCHRONY™ and OMNIsEMG™ Introduction Synchrony™ incorporates a virtual reality augmented biofeedback rehabilitation unit called the OMNIsEMG™ (sEMG) which has been specifically designed to accommodate patients with difficulty swallowing as a result of oral or pharyngeal muscle dysfunction. -
Page 8: Operation Of Synchrony
- 4 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL OPERATION OF SYNCHRONY™ To Turn ON Synchrony™ 1. The Synchrony™ cart should be plugged in when not in use so that the OMNIsEMG™ sensors remain charged. Ensure that the charging station is turned on and the sensors are plugged in properly and charging. Fully charged sensors should be available at the start of the day. -
Page 9: Adding A New Patient
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 5 - Adding a New Patient Left click the Clinician log in on the main menu Enter your Clinician ID and Password Left click the Add new patient on the Select patient category screen Patient ID/medical record# (required field) Indicate patient Gender... -
Page 10: Patient Swallowing Information
- 6 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Patient Swallowing Information Entering specific outcome oriented measures, including if the patient is non-oral feeding, swallow sores (NOMS, FOIS or MASA) and Diet levels, allowing tracking of functional outcome measurers in the patient data base. Using PENS –... -
Page 11: Managing Existing Patient Accounts
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 7 - Left click the Add clinician button From the System admin start screen, select the Manage patient’s option Left click the patient account to be edited and then left click the Manage patient’s button Enter a Clinician ID (username) for the clinician. -
Page 12: Deleting A Patient From The Database
- 8 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Deleting a patient from the database A patient may be deleted from the database at any time. This process deletes all of the patient’s records. A warning message will appear confirming the requested deletion. Choose trial type The type of trial to be performed is selected from this menu. - Page 13 SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 9 - After selecting Pharyngeal assessment, be prepared to enter the electrode placement, bolus type and volume from the drop down boxes at Drop down boxes and comment box the top of the screen. A comment box is also available to enter comments or notes as needed Electrode placement, bolus type and...
- Page 14 - 10 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Event markers and Period markers can be added by left clicking on the associated icon or using the foot pedals (L, R, M) conclusion assessment, left click the mouse over the Stop recording button or depress the M (middle foot pedal) After recording has stopped, the Event or Period markers can be...
-
Page 15: Performing An Exercise
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 11 - Performing an Exercise In addition to the patient assessment, Synchrony™ also provides the ability to perform OMNIsEMG™ guided exercises through a series of biofeedback based visualizations. Each exercise can be tailored to the needs of the patient through the use of exercise parameters. - Page 16 - 12 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Pre-exercise results After the resting baseline and three (3) exercise peaks have been captured, recording can be “Stopped”. This advances to the selected exercises pre-exercise results dashboard where results can be reviewed and or edited prior to launching the exercise session.
- Page 17 SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 13 - Selecting an exercise visualization Exercise visualizations are the virtual background the patient will see when performing the exercise. Select the visualization appropriate for the patient and double click on the selection to advance. Each exercise visualization has a series of parameters that can be adjusted to suit the needs of the...
- Page 18 - 14 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Manual timing – this setting requires the user to manually stop and end the exercise. Treatment time – this setting allows the exercise to be setup to end after a pre-set amount of time. The treatment time can be from 30 seconds to 10 minutes.
- Page 19 SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 15 - Additional Exercise Parameters (Kangaroo Pharyngeal) Typical swallow – this setting presents the intensity target (coin) at a level consistent with the pre-exercise high target Effortful swallow – this setting presents the intensity targets (coins) at a level consistent with the pre-exercise low and high target Mendelsohn maneuver - this setting presents the intensity targets (coins) at a level consistent with the pre-exercise high target.
-
Page 20: Printing And Reports
- 16 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Printing and Reports Patient assessment and exercise data can be printed to the screen, then to paper and/or saved to an external USB flash drive. In addition, any session data that is saved to a patient account can be printed at any time until that patient account is archived after discharge. - Page 21 SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 17 - Swallow scores – provides an at- a-glance view of the patients functional outcome measures of selected swallowing metrics (NOMS, FOIS or MASA) Non- oral feeding status and Diet level Treatment progression – views of swallow exercise metrics including bolus, type and volume and exercise type repetitions...
-
Page 22: Electrode Setup - Connecting The Semg Sensors
- 18 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Electrode Setup – Connecting the sEMG Sensors Remove OMNIsEMG™ sensor from charging station and swipe over the magnet on the charging station to activate (slowly flashing white LED = active). Snap the lead wires into the sensor and then remove the electrode patch from the plastic liner card. -
Page 23: Infection Control Equipment And Principles Of Use
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 19 - INFECTION CONTROL EQUIPMENT AND PRINCIPLES OF USE Definitions Barrier Film – One-time use, disposable plastic film for use over touch/operator surfaces of equipment to reduce risks of cross-contamination and need for high level disinfection of equipment between patients. ... - Page 24 - 20 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Use of Barriers – Intermediate Level Disinfection The use of an all-purpose barrier film provides surface protection from cross-contamination resulting from a variety of applications. This precaution should be used whenever dealing with non-intact skin or the chance of coming in contact with bodily fluids.
-
Page 25: Technical Specifications
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 21 - TECHNICAL SPECIFICATIONS OMNIsEMG™ Transmitter / Sensor Geometry Variable Standard sensing electrode with clip connection Electrodes Rechargeable lithium ion Battery Dimensions 17mm x 36mm x 8mm 9g battery included Weight ISM band 2.4GHz (standard IEEE802.15.4) Frequency 10G Ohms Input Impedance... -
Page 26: Troubleshooting
- 22 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL TROUBLESHOOTING Service Center For repair or service of ACP Products and accessories, please call (800) 350-1100 and follow the prompts. Normal hours of operation are 6:00am to 5:00pm Pacific Standard Time. After hours, please leave a message and a technician will return your call during the next scheduled workday. -
Page 27: Standard Limited Product Warranty
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 23 - STANDARD LIMITED PRODUCT WARRANTY The warranty information provided in this section is applicable only to products purchased from ACP, directly or through an authorized dealer. This section does not apply to leased products. The terms of maintenance and repair of any leased products are detailed in the separately executed agreement between the parties. - Page 28 - 24 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL Return of Defective Equipment Any Equipment returned to the ACP Service Center under warranty coverage must have the Warranty coverage validated and must receive authorization from ACP Customer Service prior to being received at the Service Center. Shipping charges, insurance, and any other costs incurred in sending product to ACP Service Center is the responsibility of the customer and will not be refunded.
-
Page 29: Synchrony™ And Omnisemg™ Accessories
SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL - 25 - SYNCHRONY™ AND OMNIsEMG™ ACCESSORIES ITEM ITEM NO. DESCRIPTION ™ Synchrony Synchrony™ incorporates a virtual reality augmented biofeedback rehabilitation unit 300800A called the OMNIsEMG™ which has been specifically designed to accommodate patients with difficulty swallowing as a result of oral or pharyngeal muscle dysfunction. - Page 30 - 26 - SYNCHRONY™ PROGRAM EQUIPMENT USER MANUAL SYNCHRONY™ AND OMNIsEMG™ ACCESSORIES (cont.) ITEM ITEM NO. DESCRIPTION Electrodes, Face & Neck – PENS w/snap, 74921 10packs/box 34184 Electrodes, Pharyngeal sEMG, 1-Use 15/box 61879 Electrodes, Oro-Facial, sEMG, 1-Use 15/box Pad, Electrode Skin Prep Pad w/ Pumice 69854 100/box 34774...


Need help?
Do you have a question about the ACP Synchrony and is the answer not in the manual?
Questions and answers