Summary of Contents for Devon extriCARE 2400
- Page 1 Negative Pressure Wound Therapy System extriCARE 2400 ™ Operating Manual www.devonmedicalproducts.com 866.446.0092 IFU30.0003 Rev. A 20121024...
- Page 2 1. Introduction extriCARE™ 2400 Negative Pressure Wound Therapy Pump System is a portable, battery-powered pump intended to generate negative pressure or suction to remove wound exudates, infectious material, and tissue debris from the wound bed which may promote wound healing. The extriCARE™...
-
Page 3: Symbol List
2. Symbol List Warning/Caution: See instructions for use Single Use Only Date Of Manufacture Type B. Applied Part. Internally powered electrical device Keep Dry Serial Number Prescription Use Only Power Switch Manufacture Lot Number Biohazard Class II Equipment Waste Electrical Goods Recycled... -
Page 4: Device Specifications
3. Device Specifications DIMENSIONS: Length: 3.35” (8.5cm) Height: 5.67” (14.4cm) Width: 1.46” (3.7cm) WEIGHT: 8.6 oz. MOBILITY: Portable. Carrying case available. BATTERY TYPE: Lithium (rechargeable), Model number: 623759P,1500mAh, 3.7V AC/DC ADAPTER: Input: 100-240 V, 50/60 Hz, 0.4A; Output: 5V, 1.5A VACUUM MODES: Continuous or Intermittent OPERATING CONDITIONS:... -
Page 5: Indications For Use
4. Accessories AC/DC Adapter: Input: 100 – 240V, 50/60Hz, 0.4A. Output: 5.0V, 1.5A. Tubing set: 1.8m tubing with a luer-lock connector on one end preattached. A clamp is also attached to the tubing. Canister: Available in 100, 250, and 400cc configuration. Y-Connector: A Y Bridging Kit (EC2400-Y) is available so the device can be used on multiple wound sites. -
Page 6: Contraindications For Use
Review this manual prior to using the extriCARE™ 2400 Negative Pressure Wound Therapy Pump System. If clarification is needed, contact technical personnel or Devon Medical Products at 866-446-0092 prior to use. Additional questions can be immediately addressed as well. • Do not use the extriCARE™... - Page 7 8. Precautions 8.1) Be aware for any of the following conditions: There are additional conditions to take into account before using Negative Pressure Wound Therapy, such as: BLEEDING: There is a risk of bleeding/hemorrhaging with negative pressure wound therapy. If hemostasis cannot be achieved, if the patient is on anticoagulants or platelet aggregation factors, or if the patient has friable blood vessels or infected vascular anastomosis, he or she may have an increased risk of bleeding;...
- Page 8 8.1) Be aware for any of the following conditions (continued): INFECTION: Infected wounds and osteomyelitis pose significant risks for Negative Pressure Wound Therapy. If untreated osteomyelitis is present, therapy should not be initiated. If the wound is infected, it should be closely monitored and dressings should be changed frequently. Additionally, to reduce the risk of transmission of infectious agents, standard precautions should be taken when handling or working with therapeutic parts or equipment.
- Page 9 8.2) Prior to Therapy • Patient should be assessed and measures should be taken to optimize and stabilize their medical condition. Nutrition, medication, blood glucose, blood pressure, and circulation as well as other medical issues should be addressed. • The wound should be recently debrided by whatever measure is appropriate and the amount of necrotic tissue should be minimized.
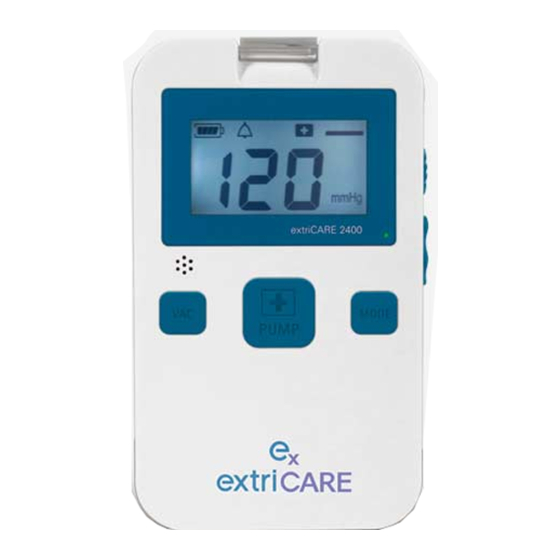
- Page 10 9. Features 9.1) Defined Features 4.) Canister Clip 3.) Battery Power 5.) Mode Symbol 10.) Power Switch 1.) LCD Screen 7.) Power Plug 9.) SET Button 6.) Mode Button 2.) Connection Tubing 8.) Pump Button LCD SCREEN: Indicates the pump operating pressure and displays symbols (also features a blue backlight).
- Page 11 9.2) Alarm Features/Troubleshooting In order to assure proper patient compliance, the extriCARE 2400 system is equipped with both audio and visual alarms for all the errors listed in the chart below. To disengage the alarms: 1. Audio alarms can be muted by pressing any button on the device front plate. 2.
- Page 12 9.2) Alarm Features/Troubleshooting (continued) Error Type Cause/Description Audio Alarm Visual Alarm System Status Suggested Features Features Mitigation Air Leakage There are many potential sources of leaks (incomplete seal between bandage and Error skin, improper connection between tubing, canister leakage, etc.) The alarms have been divided into two categories: a) Minor Pump unable...
- Page 13 10. Instructions for Use 10.1) Dressing and Canister Application The clinician may loosely place extra gauze dressing material into areas of undermining and tunneling. The decision type of gauze material used is based on clinician preference. Document the amount of additional packing material used. Dressings should be changed as needed.
- Page 14 10.1) Dressing and Canister Application (continued) When using on a venous or other leg ulcer: • Edema control must continue during wound treatment. • Consider lower pressures when applied over fragile skin. When applying the dressing over toes: • A thin layer of petroleum jelly or other oil based ointment should be applied to nails.
-
Page 15: Operating The Device
10.2) Operating the Device LCD Display POWER ON/OFF: To Power on the device, push the POWER SWITCH on the right side of the device downward. The device should then turn on. Push the POWER SWITCH upward to turn device off. CONTROL PRESSURE: Holding down the SET key for two seconds will initiate the procedure for setting the pressure. -
Page 16: Maintenance And Replacement Parts
Use only a Devon Medical Products approved battery. If the device will not be in use for an extended period of time, the battery should be maintained by... -
Page 17: Warranty Information
Operation Manual. THERE ARE NO OTHER WARRANTIES THAN THOSE EXPRESSLY STATED HEREIN. TO THE EXTENT PERMITTED BY LAW, DEVON MEDICAL PRODUCTS DOES NOT MAKE ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE AS TO ANY PRODUCT OR DEVICE, WHETHER OR NOT THAT PRODUCT OR DEVICE IS COVERED BY ANY EXPRESS WARRANTY CONTAINED HEREIN. -
Page 18: Product Classification
Appendix 1 Product Classification: • According to the type of protection against electrical shock, this device is classified as a Class II Equipment, and Type B Equipment that is powered by an external electrical power source. • According to the degree of protection against harmful ingress of water this system is classified as Ordinary Equipment (IPXO: without protection against ingress of water) •... -
Page 19: Guidance And Manufacturer's Declaration - Electromagnetic Immunity
Appendix 2 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity model extriCARE™ 2400 is intended for use in the electromagnetic environment specified below. The customer or the user of the model extriCARE™ 2400 should assure that it is used in such an environment. Immunity Test IEC 60601 Test Level Compliance Level... - Page 20 model extriCARE™ 2400 is intended for use in the electromagnetic environment specified below. The customer or the user of the model extriCARE™ 2400 should assure that it is used in such an environment. Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment Guidance Portable and mobile RF...
- Page 21 Recommended separation distances between portable and mobile RF communications equipment and the model extriCARE™ 2400. model extriCARE™ 2400 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of model extriCARE™ 2400 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the...
- Page 22 Wound Therapy for Optimal Wound Healing: A focused Review of the Literature. Ostomy Wound Management 2011;57(4):44-54 Devon Medical Products extriCARE™ Negative Pressure Wound Therapy System: extriCARE™2400 Operating Manual. 2011 Dini V. Miteva M.et al. Immunohistochemical Evaluation of Venous Leg Ulcers Before and After Negative Pressure Wound Therapy .Wounds...
- Page 23 References (continued) FDA 510K premarket notification. Devon Medical Products extriCARE™ 2400. Class II device. January 1, 2012 FDA Consumer Health Information. Negative Pressure Wound Devices Draw FDA Notice, Advice. December 2009 FDA. Medical Device Safety. Alerts and Notices. FDA Preliminary Public Health Notification: Serious Complications Associated with Negative Pressure Wound Therapy Systems.
- Page 24 www.devonmedicalproducts.com 866.446.0092...




Need help?
Do you have a question about the extriCARE 2400 and is the answer not in the manual?
Questions and answers