Advertisement
This product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization (EUA).
Please read all the information in this instruction for use before performing the test.
For use with anterior nasal swab specimens.
For In Vitro Diagnostic (IVD) Use Only.
Download App & Open App
Scan the QR code to download the "iHealth COVID-19 Antigen Rapid Test" App through your smartphone (iOS12.0+, Android 6.0+).

For a full list of compatible smartphones visit: https://ihealthlabs.com/pages/support-ICO3000
Register and Log into The App
Watch Video in App
Each step has a corresponding instructional video in the App. Watch the video and perform the test according to the instructions.
Step by Step Instructions
Prepare Materials
You may have Test Set 1 OR Test Set 2 in the package. Please follow proper steps based on the specific set you received.
Test Set 1: Open the package, take out the COVID-19 Test Card in Pouch, the Tube pre-filled with the extraction solution and the Swab. When you are ready to proceed with the test, open the foil pouch of the COVID-19 Test Card.

1 COVID-19 Test Card in Pouch

1 Pre Filled Tube

1 Swab
Please go directly to Step 2 Collect Sample.
Test Set 2: Open the package, take out the COVID-19 Test Card in Pouch, empty Tube, sealed Solution and the Swab. When you are ready to proceed with the test, open the foil pouch of the COVID-19 Test Card.

1 COVID-19 Test Card in Pouch

1 Empty Tube and 1 Sealed Solution

1 Swab
Please look carefully, there are two Edges on the empty tube. Then squeeze the sealed solution completely into the empty tube.





Please confirm the liquid level with or above Edge 2, then go to Step 2 Collect Sample.

Note:
It is acceptable if the liquid level is above Edge 2. However, please do not proceed with this test, if the liquid level is below Edge 2, as this may result in false or invalid results.
Collect Sample
- Remove the swab from its package, being careful not to touch the tip of the swab. Please keep the swab package for later use.
![iHealth - COVID-19 Antigen Rapid Test - Collect Sample Step 1 Collect Sample Step 1]()
![iHealth - COVID-19 Antigen Rapid Test - Collect Sample Step 2 Collect Sample Step 2]()
- Gently insert the entire absorbent tip of the swab (usually 1/2 to 3/4 of an inch) into your nostril.
![iHealth - COVID-19 Antigen Rapid Test - Collect Sample Step 3 Collect Sample Step 3]()
Note:
With children, the maximum depth of insertion into the nostril may be less than 3/4 of an inch, and you may need to have a second person to hold the child's head while swabbing. - Firmly and slowly brush against insides of nostril in a circular motion against the nasal wall at least 5 times. Take at least 15 seconds to collect the specimen and be sure to collect any nasal drainage on the swab. Using the same swab, repeat the same sample collection procedure for the other nostril. Be sure to brush BOTH nostrils with the SAME SWAB.
![iHealth - COVID-19 Antigen Rapid Test - Collect Sample Step 4 Collect Sample Step 4]()
Right Nostril
![iHealth - COVID-19 Antigen Rapid Test - Collect Sample Step 5 Collect Sample Step 5]()
Left Nostril
Note:
Failure to swab properly may cause false negative results.
Process Sample
- Tap the tube vertically on the table and twist the large orange cap to open the tube.
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 1 Process Sample Step 1]()
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 2 Process Sample Step 2]()
- Insert the swab into the tube, touch the bottom of the tube with the swab tip, and stir at least 15 times.
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 3 Process Sample Step 3]()
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 4 Process Sample Step 4]()
- Squeeze the sides of the tube to express as much liquid as possible from the swab, and then remove the swab.
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 5 Process Sample Step 5]()
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 6 Process Sample Step 6]()
Note:
If you don't squeeze the swab, there may not be sufficient sample material to perform the test properly (i.e., potentially resulting in a false negative result). - Screw back the large orange cap, put the swab back into the package. Safely dispose of the swab and the package.
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 7 Process Sample Step 7]()
![iHealth - COVID-19 Antigen Rapid Test - Process Sample Step 8 Process Sample Step 8]()
Add Sample
Twist to open the small white cap of the tube. Add 3 drops of sample to the Sample Port of the COVID-19 Test Card. Screw back the small white cap.


Note:
A false negative or invalid result may occur if too little solution is added to the test card.
Wait 15 Minutes
Start the timer by clicking the "Start Timer" button on the App, immediately after adding sample to the Sample Port. The result will be ready in 15 minutes.

Note:
Do NOT interpret your test result until after your 15-min timer has completed, as the T line may take as long as 15 minutes to appear.
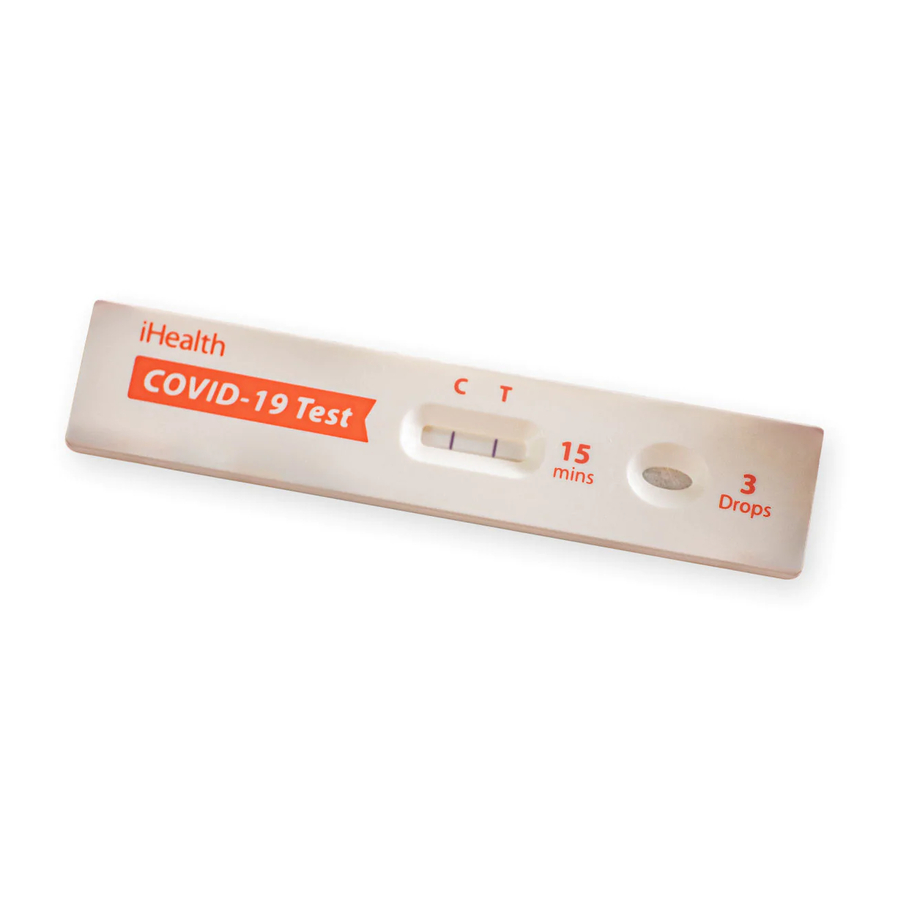
Read Result
Results should not be read after 30 minutes (Result shown at 2x magnification).
Note:
A false negative or false positive result may occur if the test result is read before 15 minutes or after 30 minutes.

Note: The T line can be extremely faint.
Test Result Explanation
Positive Result

A POSITIVE result must show BOTH a C line and a T line. A positive result means that viral antigens from COVID-19 were detected and the individual is positive for COVID-19.
Below are photos of actual positive tests. Please note that the T line may be faint.





Persons who test positive should self-isolate and seek follow up care with their physician or healthcare provider as additional testing and public health reporting may be necessary.
Negative Result

A NEGATIVE result will show ONLY a C line. A negative result means that viral antigens from COVID-19 were not detected and that the individual is presumed negative for COVID-19.
- Please note that negative results do not rule out COVID-19.
- In case of negative test result: Continue to follow all social distancing recommendations and take protective measures. If suspicions of infection persist and/or your first test is negative, repeat the test after 1-2 days and consult your healthcare provider or local COVID-19 center.
- Note: A negative result is presumptive and confirmation with a molecular assay, if necessary, for patient management may be performed. Individuals without symptoms that test negative should be tested again with at least 24 hours and no more than 48 hours between tests. Additional confirmatory testing with a molecular test for negative results may be necessary after second negative result for asymptomatic patients, if there is a high likelihood of SARS-CoV-2 infection, such as in an individual with as close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary, if there is a low likelihood of SARS-CoV-2 infection, such as in individuals without known exposures to SARS-CoV-2 or residing in communities with low prevalence of infection.
Invalid Result

If there is NO LINE, or if there is ONLY a T line, the test is INVALID. Invalid result means that the test did not function correctly. You will need to retest with a new test kit. If upon retesting, the test result is still invalid, contact your doctor or local COVID-19 center. An invalid result does not indicate if the individual did or did not have COVID-19 and should be repeated.
Dispose the Test Kit
After test is completed, dispose the kit components in trash.
Report Test Result
Report the result following the App instructions or share your test result with your healthcare provider.
In the USA:
(1) This test is intended to be used as an aid to the clinical diagnosis of a current COVID-19 infection, Do not use this test as the only guide to manage your illness.
(2) In USA - This product has not been FDA cleared or approved but has been authorized by FDA under an Emergency Use Authorization (EUA). This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other virus or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
This product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization (EUA).
Please read this instruction for use before using the test.
For use with anterior nasal swab specimens. For In Vitro Diagnostic (IVD) Use Only.
INTENDED USE
The iHealth COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of COVID-19 within the first seven (7) days of symptom onset. This test is also authorized for non-prescription home use with adult-collected anterior nasal (nares) swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first seven (7) days of symptom onset.
This test is also authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older, or adult collected anterior nasal (nares) swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
The iHealth COVID-19 Antigen Rapid Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of the SARS-CoV-2 nucleocapsid protein antigen. The antigen is generally detectable in anterior nasal swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Individuals who test positive with the iHealth COVID-19 Antigen Rapid Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
Negative results are presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual's recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19.
For serial testing programs, additional confirmatory testing with a molecular test for negative results may be necessary, if there is a high likelihood of SARS-CoV-2 infection, such as in an individual with as a close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary, if there is a low likelihood of SARS-CoV-2 infection, such as in individuals without known exposures to SARS-CoV-2 or residing in communities with low prevalence of infection.
Individuals who test negative and continue to experience COVID-19 like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow up care with their physician or healthcare provider.
Individuals should provide all results obtained with this product to their healthcare provider for public health reporting or by following the mobile application instructions for self-reporting. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC.
The iHealth COVID-19 Antigen Rapid Test is authorized for non-prescription self-use and/or, as applicable for an adult lay user testing another person aged 2 years or older. The iHealth COVID-19 Antigen Rapid Test is only for use under the Food and Drug Administration's Emergency Use Authorization.
FREQUENTLY ASKED QUESTIONS
Will this test hurt?
No, the nasal swab is not sharp and it should not hurt. Sometimes the swab can feel slightly uncomfortable or tickly. If you feel pain, please stop the test and seek advice from a healthcare provider.
What are the known and potential risks and benefits of this test?
Potential risks include:
-- Possible discomfort during sample collection.
-- Possible incorrect test results.
Potential benefits include:
-- The results, along with other information, can help your healthcare provider make informed recommendations about your care.
-- The results of this test may help limit the spread of COVID-19 to your family and others in your community.
What is serial testing?
Serial testing is when a single person is tested for COVID-19 more than once. Because antigen tests are less sensitive than other COVID-19 tests and false results may occur, repeated testing may identify individuals with COVID-19 more reliably than a single test. By repeating testing, it may be possible to more quickly identify cases of COVID-19 and reduce spread of infection. Additional testing with molecular COVID-19 test may be necessary, depending on your individual risk factors and test results. It is important that you work with your healthcare provider to help you understand the next steps you should take. Serial testing (i.e., testing every day or every other day) is more likely to detect COVID-19, especially when you do not have any symptoms.
Serial testing (i.e., testing every day or every other day) is more likely to detect COVID-19, especially when you do not have any symptoms. Testing for asymptomatic individuals should be performed at least twice over three days, with at least twenty-four hours and no more than 48 hours between tests. You may need to purchase additional tests to perform this serial (repeat) testing.
What is the difference between an antigen and molecular test?
An antigen test, such as the iHealth COVID-19 Antigen Rapid Test, detects proteins from the virus. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests are very specific for the virus, but not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. If your test result is negative, you should discuss with your healthcare provider on whether an additional test is necessary and if you should continue isolating at home. There is a higher chance of false negative results with antigen tests than with laboratory-based molecular tests. This means that there is a higher chance this test will give you a negative result when you have a COVID-19.
How accurate is this test?
The iHealth COVID-19 Antigen Rapid Test was compared to an FDA authorized molecular SARS-CoV-2 test using fresh self-collected or parent/guardian collected anterior nasal swab specimens and healthcare provider collected NP swab specimens. Subjects 2 years or older with or without symptoms participated in this study. The iHealth COVID-19 Antigen Rapid Test correctly identified 33 out of 35 (94.3%) of symptomatic positive samples and correctly identified 102 out of 104 (98.1%) of symptomatic negative samples in this study.
Please note that the accuracy of this test may decrease the longer you have had symptoms of infection, as the amount of virus in the sample decreases. In general, molecular RT-PCR tests are more sensitive than antigen tests and may be able to more reliably detect cases with less SARS-CoV-2, the virus that causes COVID-19.
What if you test positive?
A positive test result means that antigens from COVID-19 were detected and it is very likely you currently have COVID-19. There is a very small chance that this test can give a positive result that is wrong (a false positive result).
If you test positive you should self-isolate at home per CDC recommendations to stop spreading the virus to others. Please consult the CDC recommendations regarding self-isolation at www.cdc.gov/coronavirus. Seek follow-up care with your healthcare provider immediately. Your healthcare provider will work with you to determine how best to care for you based on your test result(s) along with your medical history, and your symptoms.
What if you test negative?
A negative test result indicates no antigens for COVID-19 were detected. It is possible for this test to give a negative result that is incorrect (false negative) in some people with COVID-19 and negative results are presumptive and may need to be confirmed with a molecular test. This means that you could possibly still have COVID-19 even though the test is negative. If you receive a negative result, you should test again in 24-48 hours. If you test negative and continue to experience symptoms of fever, cough and/or shortness of breath you should seek follow up care with your healthcare provider immediately. Your healthcare provider may suggest you need another test to determine if you have contracted the virus causing COVID-19. If you are concerned about your COVID-19 status after testing or think you may need follow up testing, please contact your healthcare provider.
For other updated FAQ information, please see the company website: https://www.ihealthlabs.com
For more information on EUAs go here:
https://www.fda.gov/emergency-preparednessand-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For up-to-date information on COVID-19, please visit the CDC COVID-19 website: https://www.cdc.gov/coronavirus/2019-ncov/index.html
WARNINGS AND PRECAUTIONS
- Testing for asymptomatic individuals should be performed at least twice over three days, with at least 24 hours and no more than 48 hours between tests. You may need to purchase additional tests to perform this serial (repeat) testing.
- There is a higher chance of false negative results with home use tests than with laboratory-based molecular tests. This means that there is a higher chance this test will give you a negative result when you have COVID-19.
- Serial testing (i.e., testing every day or every other day) is more likely to detect COVID-19, especially when you do not have any symptoms.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
- This test is intended for diagnosis of coronavirus infection by detecting COVID-19 antigen but should not be used as a sole criterion for the determination of SARS-CoV-2 infection. Other laboratory tests and clinical information (signs and symptoms) should be used and considered for diagnosis.
- Do not use on anyone under 2 years old.
- Children aged 2-14 years should be tested by an adult.
- Do not use on anyone who is prone to nosebleeds or has had facial or head injury/surgery in the last 6 months.
- Do not use any test component after the expiration date which is printed on the outer packaging.
- Do not use the COVID-19 Test Card if the pouch is damaged or if the seal is broken.
- Do not reuse any test component.
- To obtain accurate results, the test must be performed as indicated in the application (iHealth COVID-19 Antigen Rapid Test) and/or Instructions for Use.
- Once the COVID-19 Test Card is removed from the pouch, perform the test as soon as possible. Use the COVID-19 Test Card within 1 hour after opening the foil pouch.
- Inadequate or inappropriate sample collection may yield false test results.
- Do not touch the tip of the swab before and after collecting the sample from the nostrils.
- Insert the swab into the tube right after taking the sample.
- Test samples immediately after collection, but no more than 4 hours after specimen collection before placement into extraction buffer or up to 2 hours after placement into extraction buffer, if kept at room temperature. Be sure to read test result after 15 minutes. Do not read results after 30 minutes.
- Be sure to read test result within 15-30 minutes.
- Do not ingest extraction liquid.
- Keep test kit and components out of the reach of children and pets before and after use.
- Avoid contact with skin and eyes.
- The reagent in the extraction liquid contains ProClin® 300 which may cause an allergic skin reaction in some people. If the solution makes contact with the skin or eye, wash/ flush with copious amounts of water. If skin irritation or rash occurs get medical advice/attention.
STORAGE AND OPERATION CONDITIONS
Store iHealth COVID-19 Antigen Rapid Test in a dry place between 36-86°F (2-30°C). Ensure all test components are at room temperature 65-86°F (18-30°C) before use. The shelf-life of the iHealth COVID-19 Antigen Rapid Test is 12 months and it is stable before the expiration date marked on the packaging.
HAZARDOUS INGREDIENTS FOR REAGENT SOLUTION
The Extraction Reagent contains potentially harmful chemicals (see table below). If the solution contacts the skin or eye, flush with copious amounts of water.
If irritation persists, seek medical advice: https://www.poison.org/contact-us or 1-800-222-1222
| Chemical Name | Harms (GHS Code) for each ingredient | Concentration |
| Triton X-100 / 9002-93-1 | Harmful if swallowed (H302) Cause skin irritation (H315) Causes serious eye damage (H318) |
0.1% |
| ProClin® 300 | Harmful if swallowed (H302) Harmful if inhaled (H332) Causes severe skin burns and eye damage (H314) May cause an allergic skin reaction (H317) |
0.05% |
Manufactured for iHealth Labs, Inc.
120 San Lucar Ct, Sunnyvale, CA 94086, USA
1-855-816-7705
www.ihealthlabs.com
Made in China

VideosiHealth COVID-19 Antigen Rapid Test - How to use (video)
Documents / Resources
References
![ihealthlabs.com]() iHealth Covid 19 Antigen Rapid Test Details and FAQs (UPC, NDC, etc.)
– iHealth Labs Inc
iHealth Covid 19 Antigen Rapid Test Details and FAQs (UPC, NDC, etc.)
– iHealth Labs Inchttp://www.cdc.gov/coronavirus
![www.ihealthlabs.com]() iHealth COVID-19 Antigen Rapid Test
– iHealth Labs Inc
iHealth COVID-19 Antigen Rapid Test
– iHealth Labs Inc![www.cdc.gov]() Coronavirus Disease 2019 (COVID-19) | CDC
Coronavirus Disease 2019 (COVID-19) | CDCContact Us | Poison Control
![www.ihealthlabs.com]() iHealth COVID-19 Antigen Rapid Test
– iHealth Labs Inc
iHealth COVID-19 Antigen Rapid Test
– iHealth Labs Inc
Download manual
Here you can download full pdf version of manual, it may contain additional safety instructions, warranty information, FCC rules, etc.
Advertisement


















Need help?
Do you have a question about the COVID-19 Antigen Rapid Test and is the answer not in the manual?
Questions and answers