Table of Contents
Advertisement
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for blipcare BP800
- Page 1 Cellular Blood Pressure Monitor Model BP800 User Manual Rev 1 2019...
- Page 2 Thank you for purchasing the blip Blood Pressure Monitor! Date of Purchase: _______________________________ Serial Number: _________________________________ Questions? Comments? Call us at +1 (877) 837-9730 Learn more at www.blipcare.com Made in China © Blipcare.com All rights reserved...
-
Page 3: Table Of Contents
Table OF CONTENTS Intended Use..................4 About Blood Pressure..............5 Button Layout and Display...............6 Setting Up...................7 How to Measure Your Blood Pressure.......... 8 Taking a Measurement..............11 Maintenance..................13 Reference to Standards..............14 FCC Statement................16 RF Statement.................. 18 EMC Guidance ………………………………………………….. Safety Statements................2 Error Messages................27 Symbols....................29 Specifications.................. -
Page 4: Intended Use
INTENDED USE This device is a non-invasive blood pressure monitor. It is intended to measure Blood Pressure (Systolic and Diastolic) and Pulse Rate in the adult population (18 years or above) at home and/or office environment. The device is intended to store the readings in the BP Monitor memory and to transmit stored data to a web server on the Internet over a cellular network for easy and efficient health record keeping and trending for up to two users. -
Page 5: About Blood Pressure
ABOUT BLOOD PRESSURE High Blood Pressure, or hypertension, is a medical condition in which the arterial The chart below is the blood pressure is elevated. High blood pressure can be classified as “primary”, standard blood pressure classifi- meaning that no medical cause can be found, or as “secondary”, meaning that it is cation published by American Heart Association (AHA). -
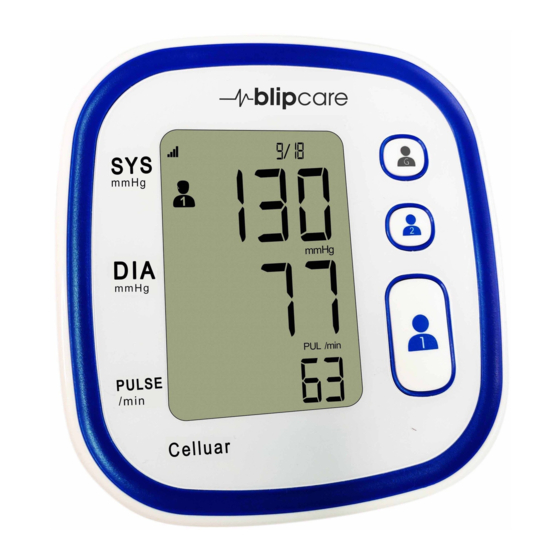
Page 6: Button Layout And Display
BUTTON LAYOUT AND DISPLAY Transmission Cellular Signal Irregular Pressing User 1, User 2 or Symbol Date Time Strength Heart Beat Guest button once will START to inflate the cuff and begin the measurement. GUEST Systolic Pressing the button again pressure while the cuff is inflating will USER 2 STOP the measurement and... -
Page 7: Setting Up
B. Once registered on blipcare.com you will be able to access your account using your login and password. Whenever you take readings, the Blood Pressure Monitor will automatically transfer your readings to your account using a cellular connection where they will be stored for future review. -
Page 8: How To Measure Your Blood Pressure
HOW TO MEASURE YOUR BLOOD PRESSURE Before taking a reading, you should sit quietly for around 10 minutes and there should be an interval of at least 5 minutes between readings. While taking a measurement, you should stay calm and relaxed, and try not to talk. This will improve the accuracy of the readings. - Page 9 PUTTING ON THE ARM CUFF Rest your left arm on a table and relax your arm. Correct cuff placement Wrap the arm cuff snugly. You should be able to fit one finger between the cuff and your arm. Correct level Make sure the cuff is at heart level.
- Page 10 PROPER POSTURE contd. Turn your left palm upward and place the edge of the arm cuff at approximately 1 inch above your elbow. The cuff should be tight enough on the arm to allow the insertion of one finger between the cuff and arm.
-
Page 11: Taking A Measurement
TAKING A MEASUREMENT 1. Push either the USER 1, USER 2 or GUEST button once to START the measurement. 2. The cuff will auto-inflate and display the pressure on the LCD. 3. The cuff will continue to inflate, display the reading upon completion and will STOP by itself. Note: The blood pressure monitor is a sensitive instrument. - Page 12 TAKING A MEASUREMENT contd. After finishing a measurement, values for systolic pressure, diastolic pressure, and pulse rate will be displayed on the LCD as shown. The blood pressure monitor will automatically transfer your measurement to the web portal via a cellular network. The cellular signal strength will be displayed.
-
Page 13: Maintenance
MAINTENANCE 1. Do not use an alcohol-based solvent or cleaning agent to clean the device. 2. Clean the device with a soft, dry cloth. 3. Do not immerse the device or cuff in water. 4. Do not bend the cuff or sleeve, and do not wrap the sleeve inside-out. 5. -
Page 14: Reference To Standards
REFERENCE TO STANDARDS Risk management EN ISO 14971:2012 / ISO 14971:2007 Medical devices - Application of risk management to medical devices EN ISO 15223-1:2016 / ISO 15223-1:2016 Medical devices. Symbols to be used with medical device labels, labelling and information to be supplied. Part 1 : Labeling General requirements User manual... - Page 15 Reference to standards contd Clinical investigation ISO 81060-2:2013 Non-invasive sphygmomanometers - Part 2: Clinical validation of automated measurement type EN 60601-1-6:2010+A1:2015/IEC 60601-1-6:2010+A1:2013 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability Usability IEC 62366-1:2015 Medical devices - Part 1: Application of usability engineering to medical devices Software life-cycle...
-
Page 16: Fcc Statement
General Communications Commission (FCC) Statement 15.21 Contains FCC ID: XMR201707BG96 This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) this device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation. - Page 17 General Communications Commission (FCC) Statement 15.21 contd. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures: -- Reorient or relocate the receiving antenna.
-
Page 18: Rf Statement
RF STATEMENT Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided in the following. -Interference may occur in the vicinity of equipment marked with -Portable and mobile RF communication equipment (e.g. cell phones) can affect Medical Electrical Equipment. -The use of accessories and cables other than those specified may result in increased emissions or decreased immunity. - Page 19 RF STATEMENT contd. -BP 800 is suitable for use in all establishments, including domestic establishments and those directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes. -Portable and mobile RF communications equipment should be used no closer to any part of BP 800 cellular blood pressure monitor, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
-
Page 20: Emc Guidance
EMC Guidance The ME EQUIPMENT or ME SYSTEM is suitable for home healthcare environments Warning:Don’t near active HF surgical equipment and the RF shielded room of an ME system for magnetic resonance imaging, where the intensity of EM disturbances is high. Warning:... - Page 21 EMC Guidance Technical description: Table 1 1. all necessary instructions for maintaining BASIC SAFETY Guidance and manufacturer’s declaration - electromagnetic emissions and ESSENTIAL PERFORMANCE with regard to Emissions test Compliance electromagnetic disturbances for the excepted service life. RF emissions Group 1 CISPR 11 2.
- Page 22 EMC Guidance Table 2 Guidance and manufacturer’s declaration – electromagnetic Immunity Immunity Test IEC 60601-1-2 Test level Compliance level Electrostatic discharge (ESD) ±8 kV contact ±8 kV contact IEC 61000-4-2 ±2 kV, ±4kV, ±8 kV, ±15 kV air ±2 kV, ±4kV, ±8 kV, ±15 kV air Electrical fast transient/burst Not application Not application...
- Page 23 EMC Guidance Table 3 Guidance and manufacturer’s declaration - electromagnetic Immunity Test Frequency(MHz) Band (MHz) Service Modulation Modulation (W) Distance (m) IMMUNITY TEST LEVEL (V/m) Radiated RF IEC61000-4-3 (Test 380-390 TETRA 400 Pulse modulation b) 18Hz specifications for GMRS 460, ENCLOSURE PORT FM c) ±...
-
Page 24: Safety Statements
SAFETY STATEMENTS Warning: No modification of this equipment is allowed 1. This device will not serve as a cure for any symptoms of heart disease. The 8. The device must only be serviced, repaired and opened by individuals at measuring data is only for reference. Always consult your physician for authorized sales/service centers. - Page 25 BATTERY SAFETY eplace the batteries whenever the Low Battery symbol shows, the display is dim or the display does not light up. To replace batteries, ● Open battery cover ● Install battery by matching polarity, ● Replace battery cover CAUTION Remove batteries if the device is not likely to be used for some time.
- Page 26 DISPOSAL Actuation of European directives 2002/95/EC, 2002/96/EC and 2003/108/EC, for reduction in use of dangerous substances in the electric and electronic device and for garbage disposal. The symbol applied on the device or its packaging means that at the end of its useful life the product must not be disposed of with domestic waste.
-
Page 27: Error Messages
ERROR MESSAGES PROBLEM SYMPTOM CHECK THIS REMEDY Replace with new batteries Batteries are exhausted. No power Insert the batteries correctly Batteries are inserted incorrectly. Display will not light up. Insert the AC adaptor tightly AC adaptor is inserted incorrectly. Display is dim or shows Low batteries Batteries are low. - Page 28 ERROR MESSAGES (continued) Communication with server failed Call Support Error message Move to a different room to improve Radio communication failure cellular signal strength Device is disabled (inactivated) Call Support for reactivation Retake the measurement. If the problem persists, contact the retailer A calibration error or our customer service department occurred.
-
Page 29: Symbols
SYMBOLS Symbol Meaning Manufacture Date Operation guide must be read 2019-08 These notes must be observed to Serial Number prevent any damage to the device. WEEE label- Environment protection. Type BF Applied part (cuff) Symbol for “DIRECT CURRENT”... -
Page 30: Specifications
SPECIFICATIONS Model BP 800 Blood Pressure Monitor Wireless transmission LTE CatM1, NBIoT, 2G Display LCD digital display Quality of Service Cellular network dependent Communication protocol MQTT Rated cuff pressure: 0mmHg~299mmHg(0kPa ~ 39.9kPa) A temperature range of :+5°C to +40°C Measurement pressure: A relative humidity range of 15% to 90%, Measuring range SYS: 60mmHg~230mmHg (8.0kPa~30.7kPa) - Page 31 SPECIFICATIONS Mode of operation Continuous operation Degree of protection Type BF applied part IP21 It means the device could protected against Protection against solid foreign objects of 12.5mm and greater, and ingress of water protect against vertically falling water drops. Battery Powered Mode: Internally Powered ME Device Classi�ication Equipment...
- Page 32 07.060.005-UI01 Rev 1 © 2019 blipcare . All rights reserved.
Need help?
Do you have a question about the BP800 and is the answer not in the manual?
Questions and answers
Message E-7
The E-7 message on the Blipcare BP800 means the device is disabled (inactivated). The user should call support for reactivation.
This answer is automatically generated