Summary of Contents for Mdoloris ANI-MR
- Page 1 ANI-MR ANI computation version: ANI OEM v2.8 ECG processing version: 3.1.0.0 Instruction for use MD/PRD/IN16.ANIMR V.04 – 14/12/2021...
- Page 3 The essential performance identified for the ANI-MR is the display of ANI index if the signal quality is good.
- Page 4 MD/PRD/IN16.ANIMR V.04 - 14/12/2021 4 | 44...
-
Page 5: Table Of Contents
Chapter 3: Setting Up ANI-MR with ANI Sensors ..........20 Unpacking and inspecting the system ............... 20 Preparation for use .................... 20 Connecting ANI Sensor V1 PLUS to the ANI-MR module ........ 21 Connecting the ANI-MR module ................ 22 Chapter 4: Operation .................... 24 The ANI-MR window .................. - Page 6 Chapter 6: Specifications ..................29 Performance Characteristics ................29 Environmental specifications ................30 Mechanical specifications .................. 31 Electrical specifications ..................31 Regulatory Symbols ..................32 Conformity ......................34 Chapter 7: Service and Maintenance ..............35 Cleaning and disinfection .................. 35 General maintenance ..................
-
Page 7: About This Manual
About this manual This manual explains how to set and use the ANI-MR. Important safety instructions for the general use of the ANI-MR are presented in this manual. Read and observe all warnings throughout this manual. The following information is a security explanation and warning. -
Page 8: Product Description
ANI index. The ANI-MR device and its sensors are designed to be used for adult patients and for paediatric patients from 12 years old. ANI-MR is intended for use under the direct supervision of a licensed... -
Page 9: Indication For Use
Drugs affecting the sinus node (atropine and other anticholinergic drugs, etc.) Side-effects No undesirable side-effects up to date, the use of the sensor with the ANI-MR may lead to undesirable side-effects. Please refer to Sensor Manual for more information. MD/PRD/IN16.ANIMR V.04 - 14/12/2021 9 | 44... -
Page 10: Warning And Cautions
Caution: read this entire manual carefully before using the ANI- MR in a clinical environment. Safety warnings and cautions WARNING : Do not use the ANI-MR if it appears or is suspected to be damaged. WARNING : Always use ANI-MR in conjunction with BeneVision monitor. - Page 11 WARNING : Not place the ANI sensors between defibrillator paddles when they are used on a patient connected to the ANI-MR. WARNING : Observe universal precautions to prevent any contact with blood or other potentially infectious materials. Contaminated materials must be handled in accordance with the facility's applicable health and safety regulations.
-
Page 12: Performance Warnings And Cautions
WARNING : The ANI-MR is not designed for use in areas containing flammable gases or vapours. WARNING : Do not pull on the patient cable, it may tear and you will no longer be able to use it. -
Page 13: Cleaning And Service Warnings And Cautions
WARNING : Do not mix disinfecting solutions (e.g., bleach and ammonia), as toxic gases may result. WARNING : Make sure the ANI-MR is installed outside the liquid projections hazard zone (e.g., Perfusion bag). WARNING : Do not autoclave the ANI-MR. Autoclaving will seriously damage the components. - Page 14 WARNING : Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the ANI-MR, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
-
Page 15: Chapter 1: Technology Overview
ANI-MR provides two averaged ANI measurements: ANIi results from the average of the raw ANI measured over the previous 56 sec, and ANIm results from the average of the raw ANI measured over the previous 176 sec. - Page 16 There are multiple ways of interpreting an ANI value. One is probabilistic, as this index has been developed in order to predict hemodynamic reactivity during nociceptive stimulation. When surgical stimulation was constant, all hemodynamic reactivity episodes (20% increase of heart rate or systolic blood pressure compared to a reference) were associated with a decreased ANI up to 10 min beforehand.
-
Page 17: Chapter 2: System Description
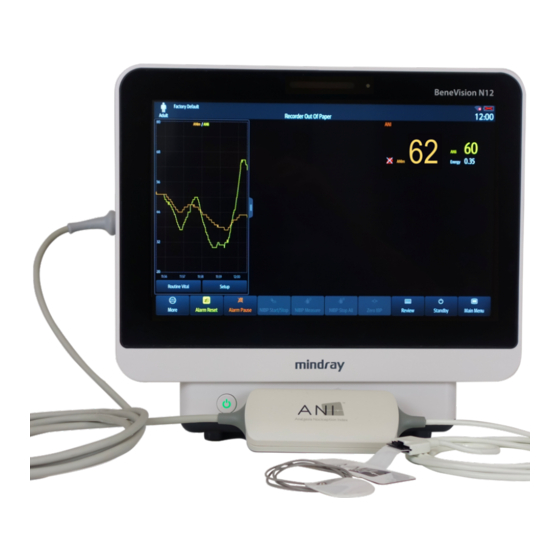
• ANIi (Instantaneous) • ANIm (Averaged) • Energy The ANI-MR is compatible with the N22/N19/N17/N15/N12/N12C BeneVision monitor from Mindray. For more information about the Mindray monitor, please read Operator’s Manual for BeneVision monitor. MD/PRD/IN16.ANIMR V.04 - 14/12/2021... -
Page 18: Mindray Ani Single Slot Communication Module
ANI-MR Connection to the ANI Sensor V1 PLUS Connection to Mindray monitor The ANI-MR computes the ANI with signals acquired from the ANI Sensors. In turn, these measurements are displayed on the BeneVision monitor. MD/PRD/IN16.ANIMR V.04 - 14/12/2021 18 | 44... -
Page 19: Ani Sensor V1 Plus
ANI Sensor V1 PLUS NOTE: The ANI-MR has been designed to work with specific disposable sensors. It is inadvisable to use another kind of electrode. The sensors can adhere to the skin for a maximum period of 24 hours. The shelf life of the sensors is indicated on the packaging. -
Page 20: Chapter 3: Setting Up Ani-Mr With Ani Sensors
Chapter 3: Setting Up ANI-MR with ANI Sensors For initial use of ANI-MR module, the following setup instructions must be followed. Unpacking and inspecting the system Remove the components from the shipping box and examine them to find signs of shipping damage. -
Page 21: Connecting Ani Sensor V1 Plus To The Ani-Mr Module
Connecting ANI Sensor V1 PLUS to the ANI-MR module Position the sensor as describe on the picture below. Sensor positioning Connect the sensor to the sensor cable. Before connecting, carefully align the notches of the ANI Sensor V1 PLUS on the connection sheet to make the pins correspond perfectly with the patient cable. -
Page 22: Connecting The Ani-Mr Module
Connecting the ANI-MR module Identify the communication connector of the module, as illustrated in the image below. Insert firmly the ANI-MR connector into the Mindray ANI single slot communication module port, itself inserted on the BeneVision monitor. MD/PRD/IN16.ANIMR V.04 - 14/12/2021... - Page 23 The ANI-MR module is now activated. This is verified when the ANI-MR parameters are displayed on the BeneVisionTM monitor. For more information on the ANI-MR window, please read The ANI- MR window section. MD/PRD/IN16.ANIMR V.04 - 14/12/2021 23 | 44...
-
Page 24: Chapter 4: Operation
Chapter 4: Operation The following sections describe how the ANI-MR information is displayed when used with the Mindray monitor, including the display details. For additional information on Mindray monitor, please read Operator’s Manual for BeneVision monitor. The ANI-MR window When an ANI-MR is connected to BeneVision... -
Page 25: Mode Of Operation
All parameters windows can be configured using the “Setup” feature, which is accessible by pressing the “Setup” button in the main menu or in the ANI window. For more information, please read Operator's Manual for BeneVision monitor. Mode of operation Once the module is connected to the patient with the sensor and to the BeneVision monitor the calculation algorithm starts automatically. - Page 26 ANI index We have developed calculation algorithms based on the amplitude measurement of the respiratory modulation of RR interval time series. A continuous index is displayed (each basic measurement is performed on 64 seconds of data with a sliding window every second) that reflects the parasympathetic tone of the patient.
-
Page 27: Chapter 5: Troubleshooting
Chapter 5: Troubleshooting To troubleshoot issues with BeneVision monitor please read the Operator's Manual for BeneVision monitor. To troubleshoot issues with the sensors, please read the Instruction for Use of the ANI Sensor V1 PLUS. Alert information Possible cause Solution •... - Page 28 ANI No Cable • • ANI-MR Disconnect disconnected from Mindray reconnect the BeneVision ANI-MR device • • Possible ANI-MR initialization software crash for a new patient will begin • • ANI has unloaded The ANI single Reconnect successfully slot module...
-
Page 29: Chapter 6: Specifications
Chapter 6: Specifications Performance Characteristics ANI index display range [0;100] ANI index display resolution 1 point ANI index precision tolerance +/- 2 points ANI index refresh rate 1 second Energy Energy display resolution [0 ;0.01] Energy useful range [0.05 ;2.5] ANI Input signal ANI input signal rate useful range [30;... -
Page 30: Environmental Specifications
Environmental specifications Operating Conditions Temperature at ambient humidity 5°C to 40°C Humidity 10 % to 95 % Atmospheric pressure 700 hPa to 1060 hPa Storage Conditions Temperature at ambient humidity -20°C to 60°C Humidity 10% to 95% Atmospheric pressure 700 hPa to 1060 hPa MD/PRD/IN16.ANIMR V.04 - 14/12/2021 30 | 44... -
Page 31: Mechanical Specifications
Mechanical specifications Module without cable Width 54 mm Length 116 mm Thickness 22 mm Cable specifications Patient cable length Communication cable length Weight 250g Electrical specifications Voltage Input +5 VDC +/- 10% Maximum current draw 200 mA Input voltage ripple <100 mV For frequencies <100kHz MD/PRD/IN16.ANIMR V.04 - 14/12/2021... -
Page 32: Regulatory Symbols
Regulatory Symbols The following symbols are on the product hardware or packaging Symbole Description Symbole Description Date of Manufacturer manufacture CE marking Serial logo number Catalogue Lot number reference Defibrillation- Refer to proof type instruction CF applied manual part /booklet Caution: Federal law restricts this... - Page 33 Temperature Keep away limits from rain Atmospheric Humidity pressure limits limits Needs special IP2X classification waste disposal Medical device MD/PRD/IN16.ANIMR V.04 - 14/12/2021 33 | 44...
-
Page 34: Conformity
Conformity Safety conformity IEC 60601-1 :2005 + AMD1 :2012 EMC conformity IEC 60601-1-2, class B Safety Classification according to IEC 60601-1 Type of Protection Class II Degree of Protection against Electric Shock CF-type Degree of Protection against liquid inlet IP 2X according to IEC 60601-1 Mode of Operation Continuous... -
Page 35: Chapter 7: Service And Maintenance
Cleaning and disinfection ANI-MR is a reusable non-sterile device. The cleaning of the ANI-MR should be performed when traces of dirt are visible on the device and at regular intervals and/or in accordance with hospitals policy, as well as local and governmental regulations. -
Page 36: Repair Policy
An authorized after sales service must perform warranty repair and service. Do not use malfunctioning equipment. Have the instrument repaired. The ANI-MR cannot be repaired by the user, any attempt at repair by the user will void the warranty. Please clean the contaminated and/or dirty equipment before returning it, following the cleaning procedure described in the Cleaning and disinfection section. -
Page 37: Warranty
Warranty MDoloris Medical Systems warrants to the initial purchaser that the ANI-MR will be free from defects in workmanship or materials, when the given normal, proper, and intended usage for a period of two years (“warranty period”) from the date of its initial shipment to the customer. -
Page 38: Software License Agreement
This warranty is the sole and exclusive warranty for MDoloris Medical Systems products, it extends only to the purchaser, and is expressly in lieu of any other expressed or implied warranties including without limitation any warranty as to merchantability or fitness for a particular purpose. - Page 39 RESTRICTIONS: you shall not transfer the Licensed Software in any manner from the System to any other computer or system without the prior written consent of MDoloris Medical Systems. You shall not distribute copies of the Licensed Software or its related documentation to others.
- Page 40 In no event shall MDoloris Medical Systems be liable to you (a) for any incidental, consequential, or indirect damages (including damages for loss of business profits, business interruption, loss of business information, and the like) arising out of the use of or inability to use any...
- Page 41 In this respect, MDoloris Medical Systems fulfils its obligations concerning the end-of-life of the ANI-MR that it places on the market by financing the WEEE Pro recycling system that collects and recycles free of charge (For more...
- Page 42 You can check that the manual you have available is up to date. MD/PRD/IN16.ANIMR V.04 - 14/12/2021 42 | 44...
- Page 44 www.mdoloris.com...






Need help?
Do you have a question about the ANI-MR and is the answer not in the manual?
Questions and answers