Table of Contents
Advertisement
Quick Links
DIGITAL AUTOMATIC
WRIST BLOOD PRESSURE MONITOR
INSTRUCTION MANUAL
MODEL No.: MD2200
Version: 1
TABLE OF CONTENTS
INTRODUCTION...............................................................................1
COMPLIANCE...................................................................................1
SYMBOLS........................................................................................2
IMPORTANT NOTES.....................................................................3-5
DEVICE DESCRIPTION.....................................................................6
BATTERY INSTALLATION.................................................................7
SETTING DATE AND TIME................................................................8
BEFORE TAKING A MEASUREMENT................................................ 9
APPLYING WRIST CUFF...................................................................9
SELECT USER................................................................................10
PERFORMING BLOOD PRESSURE MEASUREMENT......................11
RECALL AVERAGE AND PREVIOUS MEASUREMENT DATA...........12
DELETE MEASUREMENT DATA......................................................13
WHO CLASSIFICATION INDICATOR............................................... 14
ABOUT IRREGULAR HEARTBEAT ...................................................15
ABOUT BLOOD PRESSURE............................................................15
TROUBLESHOOTING......................................................................16
TECHNICAL SPECIFICATION.......................................................... 17
APPENDIX I...............................................................................18-19
APPENDIX II..............................................................................20-21
APPENDIX III..................................................................................22
APPENDIX IV..................................................................................23
Advertisement
Table of Contents

Summary of Contents for G-Lab MD2200
-
Page 1: Table Of Contents
DIGITAL AUTOMATIC COMPLIANCE...................1 WRIST BLOOD PRESSURE MONITOR SYMBOLS..................2 INSTRUCTION MANUAL IMPORTANT NOTES..............3-5 DEVICE DESCRIPTION..............6 MODEL No.: MD2200 BATTERY INSTALLATION..............7 SETTING DATE AND TIME..............8 BEFORE TAKING A MEASUREMENT..........9 APPLYING WRIST CUFF..............9 SELECT USER................10 PERFORMING BLOOD PRESSURE MEASUREMENT......11 RECALL AVERAGE AND PREVIOUS MEASUREMENT DATA...12 DELETE MEASUREMENT DATA............13... -
Page 2: Introduction
INTRODUCTION COMPLIANCE Thank you for purchasing the G.LAB MD2200 Wrist Blood Pressure This device conforms to European Medical Device Directive Monitor. 93/42/EEC. The device is easy-to-use and good for home users and healthcare professionals. It applies non-invasive oscillometric method which... -
Page 3: Symbols
SYMBOLS The following symbols are used in this instruction manual, or appear on the device, accessories and packaging. To assure correct use of the device, basic safety measures should always be followed including the warnings and cautions listed in this instruction manual. Symbols Function / Meaning Symbols... -
Page 4: Important Notes
IMPORTANT NOTES DO NOT use this device on newborns, infants, children, If you have any of the following medical conditions, you may toddlers or persons who cannot express their intentions. The get an inaccurate reading with the device. Please consult your device is designed for use on adults only. - Page 5 IMPORTANT NOTES Do not use the device where high frequency surgical Do not subject the device to strong knocks (e.g. dropping the equipment, magnetic resonance imaging (MRI), computerized unit on the floor), extreme in temperature, high humidity, direct tomography (CT) scanner or X-ray machine is operating. sunlight, dust or chemicals.
- Page 6 IMPORTANT NOTES Do not wrap the cuff around body parts other than your upper Do not place the arm cuff over heavy clothing (e.g. a jacket or left arm. Misuse represents a risk to your health. sweater sleeve) as the blood pressure monitor will not be able to take a proper measurement and there is an elevated danger Packaging materials are a deadly hazard for children and can of acquiring hematoma or skin marks during the course of the...
-
Page 7: Device Description
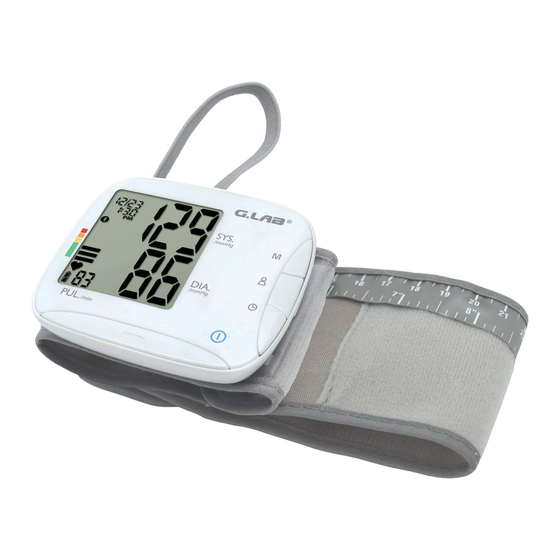
DEVICE DESCRIPTION Information on the Display : Average Data Memory Record Number and Data Display Clock Key Date User Selection Key Time Memory Key Systolic User 1 Pressure User 2 Severe Hypertension Moderate Hypertension Mild Hypertension High Normal Normal Optimal Diastolic Pressure Pressure Bar... -
Page 8: Battery Installation
BATTERY INSTALLATION Battery Level Indicator 1. Remove the battery cover. symbol on the display indicates that battery level is normal. When battery level is getting low, symbol and “E6” will appear on the display. Replace all used batteries with new batteries. NOTE: Batteries may cause a choking hazard to children. -
Page 9: Setting Date And Time
SETTING DATE AND TIME (A) When new batteries are installed 1. “12h” will blink on the display. 2. Press M key to select “12h” or “24h” time display format. 3. Press key to confirm and then "YEAR" will start to blink. 4. -
Page 10: Before Taking A Measurement
BEFORE TAKING A MEASUREMENT APPLYING WRIST CUFF • Before using the device, check your wrist circumference and make 1. Bare your left wrist. Make sure that the sure it matches the wrist cuff circumference range. blood circulation in your arm in not •... -
Page 11: Select User
SELECT USER The device has a memory capability to store the measurement data for 2 users – User 1 and User 2. Every time you complete the measurement, the device automatically stores the measurement result. 1. Press key to turn on the device. 2. -
Page 12: Performing Blood Pressure Measurement
PERFORMING BLOOD PRESSURE MEASUREMENT 1. Press key to turn on the device. 2. The cuff deflates to remove residue air before starting measurement. Releasing air indicator blinks on the display. 3. Memory record number appears on the display. 4. The cuff starts to inflate. It is normal for the cuff to feel tight. If you want to stop cuff inflation, press key to turn off the device and the cuff will deflate. -
Page 13: Recall Average And Previous Measurement Data
RECALL AVERAGE AND PREVIOUS MEASUREMENT DATA The device has a memory capability to store the measurement data View previous measurement data. for 2 users – User 1 and User 2. Every time you complete the 2. Press M key to view previous measurement data. measurement, the device automatically stores the measurement Corresponding memory record number and time of measurement result. -
Page 14: Delete Measurement Data
DELETE MEASUREMENT DATA There are 2 methods to delete the memory record. (A) Delete one memory record (B) Delete all memory records 1. While you are viewing previous measurement data, select the 1. Press M key to view the average measurement data. record you want to delete. -
Page 15: Who Classification Indicator
WHO CLASSIFICATION INDICATOR The World Health Organization (WHO) has established the following The WHO Classification Indicator is a feature which provides a chart as a standard to assess high blood pressure, regardless of the snapshot of your blood pressure classification based on your age. -
Page 16: About Irregular Heartbeat
ABOUT IRREGULAR HEARTBEAT [IHB] ABOUT BLOOD PRESSURE An irregular heartbeat is defined as a heartbeat that varies by 25% What Is Blood Pressure? from the average of all heartbeats during the blood pressure Blood pressure is the force exerted by blood against the walls of the measurement. -
Page 17: Troubleshooting
TROUBLESHOOTING Problem Probable Cause Correction Batteries are drained. Replace all used batteries with new batteries. Nothing appears on the display, even when the Batteries are not installed in correct Re-install the batteries with their polarities (“+” and “-“) match the power is turned on. -
Page 18: Technical Specification
TECHNICAL SPECIFICATION Model No. : MD2200 Display : LCD Display Measurement Method : Non-invasive, Oscillometric method Measurement Range : Systolic Blood Pressure: 50-250 mmHg Diastolic Blood Pressure: 30-200 mmHg Pulse Rate : 40-180 beats/minute Accuracy : Pressure : +/-3 mmHg... - Page 19 APPENDIX I The Sphygmomanometer (MD2200) is intended for use in the electromagnetic environment specified below. The customer or the user of the Sphygmomanometer (MD2200) should assure that it is used in such an environment. Conducted and radiated RF Compliance Electromagnetic environment - guidance...
- Page 20 The Sphygmomanometer (MD2200) is intended for use in the electromagnetic environment specified below. The customer of the user of Sphygmomanometer (MD2200) should assure that it is used in such an environment. Floors should be wood, concrete or Electrostatic discharge ceramic tile. If floors are covered ±...
- Page 21 APPENDIX II The Sphygmomanometer ( MD2200 ) is intended for use in the electromagnetic environment specified below. The customer of the user of Sphygmomanometer ( MD2200 ) should assure that it is used in such an environment. IEC 60601 Immunity test...
- Page 22 NOTE These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. r.m.s., before modulation is applied. The ISM (industrial, scientific and medical) bands between 0,15 MHz and 80 MHz are 6,765 MHz to 6,795 MHz;...
- Page 23 APPENDIX III Recommended separation distances between portable and mobile RF communications equipment and the Sphygmomanometer MD2200 MD2200 The Sphygmomanometer ( ) is intended for use in an electromagnetic environment in which radiated RF disturbances MD2200 are controlled. The customer or the user of the Sphygmomanometer (...
- Page 24 APPENDIX IV This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications.
- Page 25 Symbol for the marking of electrical and electronics devices according to Directive 2002/96/EC. The device, accessories and the packaging have to be disposed of waste correctly at the end of the usage. Please follow 5-YEAR LIMITED WARRANTY Local Ordinances or Regulations for disposal. Thank you for your recent purchase of the G.LAB®...






Need help?
Do you have a question about the MD2200 and is the answer not in the manual?
Questions and answers