Table of Contents
Advertisement
Quick Links
Table of Contents
Design Philosophy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Product Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
User Profile . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Intended Use/Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Performance Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Warnings And Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Latex Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Contents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Pump Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Symbol Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Pump Set-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Cuff Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Pump Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Single Leg Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Patient Compliance Counter Reset . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Alarm Reset . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
S Mode Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Tube Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Enabling Compliance Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Compliance Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Call For Service Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Battery Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Battery Charging Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Lithium Iron Phosphate Battery Maintenance Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Battery Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Handling Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Low Battery Alarm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Instructions for Installing/Removing/Replacing the Power Cord . . . . . . . . . . . . . . . . . . . . 17
Telescoping Bed Hanger Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Instructions for Replacing Fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
ENGLISH
1
Advertisement
Table of Contents

Summary of Contents for DJO VENAFLOW Elite
-
Page 1: Table Of Contents
Table of Contents Design Philosophy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 Function . - Page 2 VenaFlow Elite . . . . . . . . . . . . . . . . .
-
Page 3: Design Philosophy
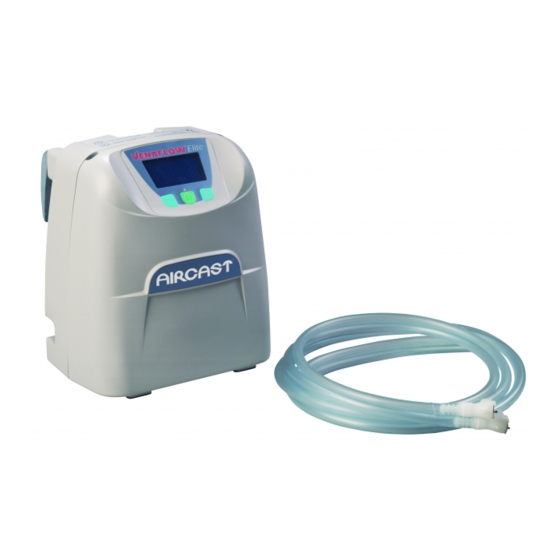
.¹ The Aircast VenaFlow Elite System leverages the same clinically proven technology as the existing VenaFlow offering, but is presented in a low profile, light-weight design . VenaFlow Elite combines two proven technologies, rapid inflation and graduated sequential compression, via our new single tube Integrated Graduated Sequential Flow System (IGSF), to accelerate venous velocity and enhance fibrinolysis . -
Page 4: Product Description
The VenaFlow Elite S-Mode System is intended as a prophylaxis for deep vein thrombosis (DVT) . The System is for Adult Use only . The VenaFlow Elite S-Mode System is designed to be used only with Aircast VenaFlow Elite Compression Cuffs . Battery operation use only with DJO, LLC battery packs . -
Page 5: Contraindications
Contraindications The VenaFlow Elite System should not be used by persons with known or suspected deep vein thrombosis, severe congestive heart failure, pulmonary edema, thrombophlebitis, severe arteriosclerosis, or active infection . Do not use on extremities which are not sensitive to pain, where cuff will interfere with gangrene, on patients with vein ligation or recent skin grafts, or extreme deformity of the leg . -
Page 6: Latex Information
. Latex Information All components of the VenaFlow Elite System are not made with natural rubber latex . All VenaFlow Elite cuffs are not made with natural rubber latex and may be placed directly against the skin or over a light compression dressing . -
Page 7: General System Information And Application
7) Battery indicator* - Indicates battery charge. 8) S Mode operation button 9) ON / OFF / RESET push button - Turns the device on or off and resets the alarms. * Note: not all VenaFlow Elite Systems will contain a battery ENGLISH... -
Page 8: Symbol Definitions
Symbol Definitions Authorized repesentative in the Power on/off button and alarm European Community reset Manufacturer Compliance monitor reset Date of Manufacturer Tube alarm Dual calf/thigh Call for service Single calf/thigh Inflation cycle indication (Bars indicate cuffs are inflating) Dual foot Type BF Equipment Single foot F2AL250V... -
Page 9: Pump Set-Up
Pump Set-up 1) Hang pump from bed frame (foot of bed), bed rail, or rest on floor or table . To use the telescoping bed hanger, press release button on the back of the device and gently pull out bed hook to the desired width . -
Page 10: Cuff Application
Cuff Application The VenaFlow Elite System will automatically detect the cuff that is attached to the device and apply appropriate pressures . You must attach the cuffs prior to powering on the device . 1) Calf cuff – Apply cuff with the tube pointed toward the foot . The aircell may be placed either on the back, side or front of the leg . -
Page 11: Pump Operation
Pump Operation 1) To turn the device on, press . The graphical display, green pump indicator lights and green light above button will turn on . 2) Once device is powered on, system will immediately enter cuff detection mode which means the system will be detecting whether or not there are cuffs attached . Display will read “Detecting Cuffs”... -
Page 12: System Alarms
. S Mode Operation When powered on, the VenaFlow Elite System will default to standard rapid inflation mode . If a slower inflation is desired, select the S mode operation button . -
Page 13: Tube Alarm
FOR SERVICE” . Additionally, the pump indicator lights on the side of the system will flash red . 2) If Call For Service alarm occurs, unplug the device and call DJO Technical support . See Customer Care Contact Information section . ENGLISH... -
Page 14: Battery Information And Operation
Battery Information and Operation Battery Operation The VenaFlow Elite can be installed with a battery upon request at the time of the initial system order . When the battery-installed system is powered on, a battery icon will appear on the right side of the graphical display beneath the leg icons . If the system does not have a battery installed, then there will be no battery icon on the graphical display at all . -
Page 15: Battery Maintenance
. Consider shipping the device back to DJO to replace the battery if you note either of the following conditions: 1 . The battery run time drops below about 80% of the original run time . -
Page 16: Low Battery Alarm
Low Battery Alarm When there is approximately 10 minutes of charge left in the battery, an audible alarm will beep for 10 seconds per minute and a battery icon will appear on the graphical display and alternate with the text "LOW BATTERY" . When there is approximately 5 minutes left, two 10 second beeps will sound per minute in addition to the graphical alert . -
Page 17: Replacement Kit Instructions
Replacement Kit Instructions Replacement kits may be ordered through customer care . Replacement Kit Instructions Instructions for Installing/Removing/ Replacing the Power Cord 1) Make sure system is powered off and unplugged . 2) Remove the screw retaining the power cord cover using a 1/16”... -
Page 18: Cleaning Instructions
NOTE: The unit was tested during the manufacturing process and is ready to be placed into service upon delivery . Factory Service When the VenaFlow Elite System requires factory service, contact Customer Service . See Customer Care Contact Information section . ENGLISH... -
Page 19: Warranty
Warranty For consigned systems: DJO, LLC will repair or replace all or part of the 30BI-S and 30BI- SB systems for material or workmanship defects for the life of the customer contract . Warranty and maintenance terms are specified in each consignment contract . -
Page 20: Ordering Information
Tube Assembly, 320 .04 cm (126 in .) Each 3008XXXL Tube Assembly, 381 cm (150 in .) Each System Replacement Parts Part # Description 3071 Bed Hanger 3072 Tube Attachment Tag 3073 Fuse For powercords, see VenaFlow Elite International Plug Ordering Matrix . ENGLISH... -
Page 21: Venaflow Elite International Plug Ordering Matrix
VenaFlow Elite International Plug Ordering Matrix Power Country Language Plug Part number Voltage Freq. United Kingdom English 230 V 50 Hz BS1363 3048 Germany German 230 V 50 Hz CEE7/7 3049 German Belgium French 230 V 50 Hz CEE7/7 3049 Dutch Austria German 230 V 50 Hz... -
Page 22: Compliance Statements
Compliance Statements Electromagnetic Compatibility (EMC) The VenaFlow Elite has been tested and found to comply with the electromagnetic compatibility (EMC) limits for medical devices to IEC 60601-1-2 . These limits are designed to provide reasonable protection against harmful interference in a typical medical installation . -
Page 23: Electromagnetic Compatibility (Emc) Tables - Rf Emissions Class A
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions The VenaFlow Elite is intended for use in the electromagnetic environment specified below . The customer or the user of the VenaFlow Elite should assure that it is used in such an environment . -
Page 24: Guidance And Manufacturer's Declaration - Electromagnetic Immunity
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The VenaFlow Elite is intended for use in the electromagnetic environment specified below . The customer or the user of the VenaFlow Elite should assure that it is used in such an environment . -
Page 25: Guidance And Manufacturer's Declaration - Electromagnetic Immunity
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The VenaFlow Elite is intended for use in the electromagnetic environment specified below . The customer or the user of the VenaFlow Elite should assure that it is used in such an environment . -
Page 26: Recommended Separation Distances Between Portable And Mobile Rf
The VenaFlow Elite is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled . The customer or the user of the VenaFlow Elite can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the VenaFlow Elite as recommended below, according to the maximum output power of the communications equipment . -
Page 27: Specifications
Specifications Size: 19 .3 cm (7 .6 in .) x 21 .6 cm (8 .5 in .) x 11 .7 cm (4 .6 in .) Weight: no battery: 1 .8 kg (4 lbs .) with battery: 2 .2 kg (4 .75 lbs .) Power input: 100 - 240~, 50/60 Hz, 75 VA Cord: Hospital grade, 4 .572 m (15 ft .) Standards: IEC 60601-1:2005, IEC 60601-1-2:2007... -
Page 28: Customer Care Contact Information
CANADA: Tel Denmark: +46 40 39 40 00 DJO Canada Email: info .nordic@DJOglobal .com 6485 Kennedy Road FRANCE: Mississauga DJO France S .A .S . Ontario Centre Européen de Fret L5T 2W4 64990 Mouguerre CANADA FRANCE Tel: +1 1866 866 5031... - Page 29 CA 92081-8553 Fax: +39 02 484 09217 U.S.A. Email: vendite@DJOglobal .com Tel: 1 800 494 3395 SOUTH AFRICA: DJO South Africa (Pty) Ltd Unit 1, Brackengate Business Park 5 on London Brackenfell, 7560 Cape Town SOUTH AFRICA Tel: +27 (0) 87 3102480 Fax: +27 (0) 86 6098891 Email: info .southafrica@DJOglobal .com...
- Page 30 DJO Export ASIA-PACIFIC: DJO Asia-Pacific Limited Unit 1905, 19/F Tower II Grand Central Plaza 138 Shatin Rural Committee Road Shatin HONG KONG Tel: +852 3105 2237 Fax: +852 3105 1444 Email: info .asia-bs@DJOglobal .com EUROPE, MIDDLE EAST, AFRICA EXPORT: DJO Benelux...
- Page 32 DJO, LLC MDSS GmbH 1430 Decision Street Schiffgraben 41 Vista, CA 92081-8533, USA 30175 Hannover, Germany 30B120 REV F - 2021-03-17...











Need help?
Do you have a question about the VENAFLOW Elite and is the answer not in the manual?
Questions and answers