Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

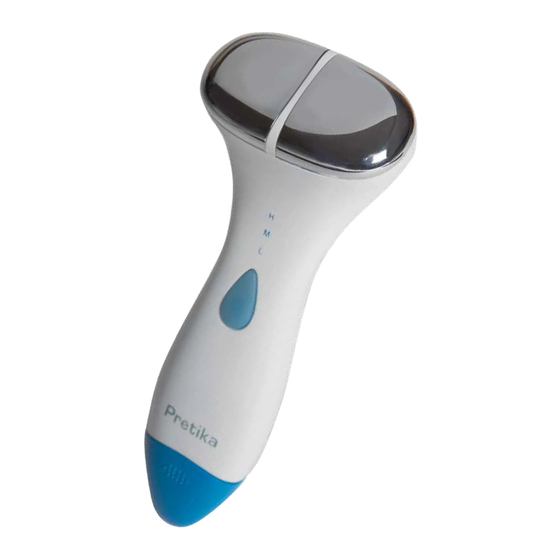
Summary of Contents for Pretika SonicLift ST280
- Page 2 For important information about safety and how to use the device, read the full Instructions for Use booklet. Contact the experts at Pretika for any assistance in helping you get started. Customer Service Contact: 1-949-481-8818 Email: customerservice@pretika.com or visit our website at www.pretika.com...
-
Page 3: Table Of Contents
Contents Package Contents and Components.....…………………….. 4 What is this Device Used For?..........…. 5 Contradictions………………………………………………………… 6 Warnings……………………………………………………..……… 7 Precautions…………………………………………………………… 8 Risks………………………………………………………………..9 Benefits……………………………………………...…………... 10 Getting Started…………………………………….......…. 11 To Charge Batteries……………………………………………..……. 11 How to Use…………………………………………………....12 Buttons & Indicator Lights…………………………………………..13 Treating Target Areas………………………………………………..14 What to Expect……………………………………………………….. -
Page 4: Package Contents And Components
Package Contents and Components Package Contents • Treatment Spheres • Hand Piece Components 3 Power Button 6 oz / 177mL Conductive... -
Page 5: What Is This Device Used For
What is Device Used For? Micro Current Device is a non-invasive facial toning device intended for at-home cosmetic use. This device delivers low level electrical micro-current impulses to strategic locations on the face to improve your facial tone. Electrical current is emitted from dual probes that are designed for optimal contact with faces of all shapes and sizes. -
Page 6: Contradictions
Contradictions Never use this device over any suspicious or cancerous lesion. Using this device over cancerous or other type of lesion can result in delaying the best medical treatment. Typical characteristics of cancerous lesions include: • Asymmetry: one half of the abnormal skin area is different than the other half •... -
Page 7: Warnings
Warnings Warning: Discontinue use if you encounter any discomfort or skin redness that lasts for more than two hours. Consult with your skincare professional or healthcare provider. Warning: Do not use if you are pregnant or breastfeeding. Warning: Do not use if you have experienced a migraine in the last two years. Warning: Do not use over thyroid gland (neck area). -
Page 8: Precautions
Precautions Stop immediately if you experience any adverse effect of have a concern regarding use. Contact your skin care professional, Doctor or healthcare provider. Use this device only for its intended use as described in this Instructions for Use Manual. Your Micro Current Device is designed for cosmetic use only and for individuals in good health. -
Page 9: Risks
Pretika Corporation nor its affiliates. HAZARD LIKELIHOOD OF HAZARDOUS... -
Page 10: Benefits
Benefits BENEFITS EFFECTS TO USER LIKELIHOOD OF BENEFIT Improve your facial tone Users of this device can obtain benefit from using it only if they read and understand all information in the user manual and follow exactly all the instructions in the user manual. Conductive Gel Ingredients Water, 1, 2 Propanediol, Acrylic Acid Polymer, Caprylyl Glycol, Methylisothiazolinone, Cellulose Polymer. -
Page 11: Getting Started
Getting Started Device Setup Getting started with Micro Current Device is easy. To Install or Replace Batteries Make sure power is in the Off position. Remove Outer battery-compartment cover by holding the device and pulling outward the lift tab. Lift and pull open Inner battery-compartment cover. Carefully pull out batteries (if replacing) and insert 3 new “AA”... -
Page 12: How To Use
Ensure the entire area being treated is saturated with the Conductive Gel. On your clean, dry face apply Pretika Conductive Gel by tapping into your skin with your fingertips. We recommend applying the Gel to each treatment area or half the face at a time, so that the Gel does not dry out. -
Page 13: Buttons & Indicator Lights
BUTTON LIGHT INDICATORS On/Off Button ON: Solid red light will appear Control Mode Button RED LIGHT: Gentle Mode GREEN LIGHT: Medium Mode BLUE LIGHT: High Mode Step 3: Glide along the natural contours of your face, in an upward motion. Each glide should last five (5) sec- onds, and should be repeated three (3) times. -
Page 14: Treating Target Areas
Treating Target Areas 3 Easy Steps Step 1 Step 2 Step 3 PREP LIFT FINISH Apply any Conductivity Glide the device over With a warm, Gel to your targeted the natural contours damp washcloth, treatment area(s). of your face. gently remove the Conductivity Gel then apply your serums and moisturizer. -
Page 15: Device Maintenance
Device Maintenance The following maintenance instructions are important to ensure that your device continues to work as it was designed. Failure to follow these instructions may cause your device to stop delivering the required dose or to stop working altogether. Cleaning Simply follow these easy instructions: Step 1: Lightly dampen a washcloth with water... -
Page 16: Trouble Shooting
Device shuts off in mid-treatment or only Check and recharge batteries. after a few treatments If you still have questions please visit www.pretika.com or of call Pretika at (949) 481-8818 for more tips and troubleshooting. Disposal of Electrical and Electronic Equipment Waste... -
Page 17: Service
Pretika Corporation in the amount of $40.00 to cover handling and postage charges and we will send a replacement unit. -
Page 18: Specifications
Know Your Device Labels & Symbols ype B equipment suitable for intentional external applied part to the patient, excluding “Direct Cardiac Application” Specifications Power Requirements Power Input: 1.5Vx3 AA alkaline batteries Voltage: 3Vdc Operating Specifications Ambient Temperature: 15 degrees C to 35 degrees C; RH: 15% to 17%, non-condensing Maximum Output Voltage Transport/Store Environmental Specifications... -
Page 19: Warranty
GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC EMISSIONS The MODEL ST280 is intended for use in the electromagnetic environment specified below. The customer or the user of the MODEL ST280 should assure that it is used in such an environment. Emissions Compliance Electromagnetic environment –... - Page 20 GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC IMMUNITY The MODEL ST263 is intended for use in the electromagnetic environment specified below. The customer or the user of the MODEL ST263 should assure that it is used in such an environment. Immunity test IEC 60601 Compliance level Electromagnetic environment –...
- Page 21 GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC IMMUNITY The MODEL ST263 is intended for use in the electromagnetic environment specified below. The customer or the user of the MODEL ST263 should assure that it is used in such an environment. Immunity test IEC 60601 test level Compliance Electromagnetic environment –...
- Page 24 Facial Toning Lift, Firm and Tone Skin...

Need help?
Do you have a question about the SonicLift ST280 and is the answer not in the manual?
Questions and answers