Table of Contents
Advertisement
Quick Links
PROFESSIONAL MEDICAL PRODUCTS
MISURATORE DI PRESSIONE LEO CON SOFTWARE
LEO BLOOD PRESSURE MONITOR
WITH SOFTWARE
TENSIOMÈTRE LEO AVEC LOGICIEL
DE LEO MEDIDOR DE PRESIÓN
CON SOFTWARE
MEDIDOR DA TENSÃO LEO COM SOFTWARE
32902 / CONTEC08A
CONTEC MEDICAL SYSTEMS CO., LTD
No.112 Qinhuang West Street, Economic & Technical Development Zone,
Qinhuangdao, Hebei Province, PEOPLE'S REPUBLIC OF CHINA
Made in China
Shanghai International Holding Corp. GmbH (Europe)
Eiffestrasse 80, 20537 Hamburg, Germany
Importato da / Imported by / Importé par / Importado por / Importado por:
Gima S.p.A.
Via Marconi, 1 - 20060 Gessate (MI) Italy
gima@gimaitaly.com - export@gimaitaly.com
www.gimaitaly.com
0123
Advertisement
Table of Contents

Summary of Contents for Contec CONTEC08A
- Page 1 CON SOFTWARE MEDIDOR DA TENSÃO LEO COM SOFTWARE 32902 / CONTEC08A 0123 CONTEC MEDICAL SYSTEMS CO., LTD No.112 Qinhuang West Street, Economic & Technical Development Zone, Qinhuangdao, Hebei Province, PEOPLE’S REPUBLIC OF CHINA Made in China Shanghai International Holding Corp. GmbH (Europe) Eiffestrasse 80, 20537 Hamburg, Germany Importato da / Imported by / Importé...
- Page 2 ENGLISH Foreword Please read the User Manual carefully before using this product. The User Manual which describes the operating procedures should be followed strictly. This manual detailed introduce the steps must be noted when using the product, operation which may result in abnormal, the risk may cause personal injury and product damage and other contents, refer to the chapters for details.
-
Page 3: Table Of Contents
ENGLISH Contents Chapter 1 Safety Precautions ............................53 1.1 Operation for AC Adapter (Separate Sale) ....................56 1.2 Operation for Battery ..........................56 Chapter 2 Main Unit ............................... 58 Chapter 3 External Interfaces ............................61 Chapter 4 Battery/AC Adapter Installation ........................63 4.1 Battery Installation ............................ - Page 4 ENGLISH Chapter 14 Installation of the Software.......................... 78 14.1 Demand of Editor............................ 78 14.2 Installation of Software ........................... 78 Chapter 15 Error Message............................... 79 Chapter 16 Troubleshooting ............................80 Chapter 17 Keys and Symbols............................82 Chapter 18 Maintenance, Cleaning and Keeping ......................83 Chapter 19 NIBP Specification ............................
-
Page 5: Chapter 1 Safety Precautions
ENGLISH Chapter 1 SAFETY PRECAUTIONS • In order to use it correctly, please read the “Safety Precautions” carefully before using it. • Operators do not need professional training, but should use this product after fully understanding the requirements in this manual. - Page 6 ENGLISH Patient Movement Measurements will be unreliable or can not perform if the patient is moving, shivering or having convulsions. These motions may interfere with the detection of the arterial pressure pulses. In addition, the measurement time will be prolonged. Cardiac Arrhythmia’s Measurements will be unreliable and may not be possible if the patient’s cardiac arrhythmia has caused an irregular heart- beat.
- Page 7 ENGLISH Please do not keep the cuff in the over-inflated state for a long time. Otherwise it may cause risk. Do not use the device in the case of there are flammable anesthetic gasses mixing with the air or nitrous oxide. Otherwise it may cause risk.
-
Page 8: Operation For Ac Adapter (Separate Sale)
ENGLISH Without our company or other approved maintenance organizations trained service personnel should not try to maintain the product. This device can only be used for one test object at a time. If the small parts on the device are inhaled or swallowed, please consult a doctor promptly. The device and accessories are processed with allergenic materials. - Page 9 ENGLISH New and old batteries, different kinds batteries can not be put off. Otherwise it may cause battery leakage, heat, rupture, and damage to Electronic Sphygmomanometer. Please don’t put wrong the positive and negative of battery. When the batteries power exhausts, replace with four new batteries at the same time.
-
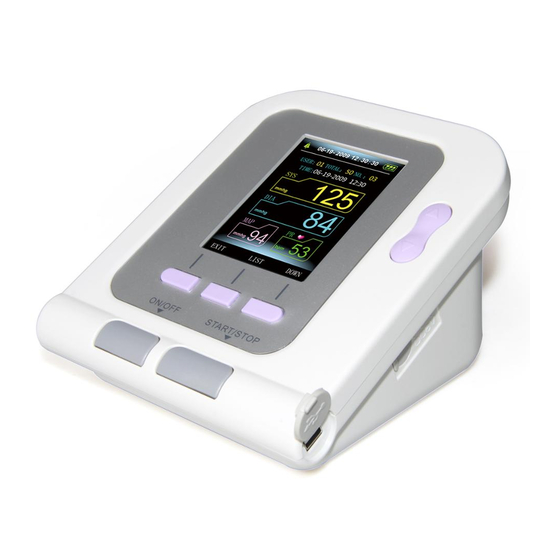
Page 10: Chapter 2 Main Unit
ENGLISH Purpose: The device apply to measure the non-invasive blood pressure and SpO (optional) of human. Record parameter value of blood pressure to provide the reference for the health care professional. Chapter 2 MAIN UNIT Display The production is in the package. Cuff plug Open the package and confirm whether the production is whole. - Page 11 ENGLISH Accessories: Specification: limb circumference 22-32 cm (middle part of upper arm), please select suited cuff when measuring children or other limb circumference. USB Data Line Software CD User Manual Optional Accessories: AC adapter Input: voltage: AC 100 V~240 V Frequency: 50 Hz/60 HZ Rated current: AC 150 mA Output: DC 6.0 V±0.2 V 1.0 A...
- Page 12 ENGLISH Note: because SpO probe measurements are statistically distributed, only about two-thirds of SpO probe measurements can be expected to fall within ±Arms of the value measured by a CO-OXIMETER. B. Pulse rate measurement Range:30 bpm~250 bpm Error: ±2 bpm or ±2% (select the larger) Resolution: 1bpm C.
-
Page 13: Chapter 3 External Interfaces
ENGLISH Note • The cuff is a consumable. Calculate by measuring 6 times a day(3 times each morning and evening), the service life of the cuff is about 1 year.(using our experimental conditions). • In order to correctly measure blood pressure, please replace the cuff in time. •... - Page 14 ENGLISH The right side of the device is USB socket and power adapter socket • USB socket ( is USB identifier) Right side • Power adapter socket ( is power socket identifier) Note All analog and digital equipment connected to this device must be certified to IEC standards(such as IEC60950: Information technology equipment-Safety and IEC60601-1: Medical electrical equipment-Safety), and all equip- ment should be connected to in accordance with the requirement of the valid version of the IEC60601-1-1 system standard.
-
Page 15: Chapter 4 Battery/Ac Adapter Installation
ENGLISH Chapter 4 BATTERY/AC ADAPTER INSTALLATION The product can use battery or AC adapter as power source. 4.1 Battery Installation 1. Demount the battery cover in the direction of the arrow. 2. Install “AA” batteries according to + - polarities. 3. -
Page 16: Usage Of Power Adapter
ENGLISH 4.2 Usage of power adapter 1. Connect the sphygmomanometer and the power adapter. Insert the power adapter plug into the power adapter socket on the right side of the device. 2. Please insert the power plug of the adapter into the AC 100 V~240 V socket. Note The device can be disconnected from the power supply network by unplugging the adapter plug. -
Page 17: Chapter 6 Setting The Date And Time
ENGLISH • At all levels interface, the three buttons correspond respectively with the text prompts below the LCD screen, pressing any button will carry on the corresponding function, eg: [MENU] [ENTER] [LIST] [USER] etc. • Up and down buttons, respectively carry on the functions of moving the cursor up and down, changing the param- eters and switching the status. -
Page 18: Chapter 7 About Unit
ENGLISH Chapter 7 ABOUT UNIT There are two units: “mmHg” and “kPa”. The default is: “mmHg”. Enter the [SYSTEM SETUP] submenu in [SYSTEM MENU], then select [UNIT] option to switch units be- tween “mmHg” and “kPa”. Chapter 8 USER SWITCH The Electronic Sphygmomanometer stores the measure results of three users automatically, and up to 100 items for every user. -
Page 19: Chapter 9 Over-Limit Prompt Function
ENGLISH Note When the [USER PREVIEW] is set to [ALL], , current user can be switched under main interface; when set to a cer- tain user, it will not be able to switch under main interface. User type can be set to adult, pediatric and neonatal three different kinds, setting method is as follows: Chapter 9 OVER-LIMIT PROMPT FUNCTION... -
Page 20: Technical Parameter Over-Limit Prompt
ENGLISH 9.2 Technical parameter over-limit prompt When power is about to exhaust and prompt is ON, the prompt will occur. This prompt can not be cancelled unless being closed or the power replaced. Chapter 10 THE USAGE METHOD OF SPHYGMOMANOMETER 10.1 Accurate Measurement Way Measurement in quiet and relaxing state. - Page 21 ENGLISH Advice Try to measure your blood pressure at the same time each day with the same arm and the same pose for consist- ency. The high and low location of cuff will cause changes in measure results. Do not touch the sphygmomanometer, cuff and windpipe during measure. Measurements should be taken in a quiet place and the body relax.
-
Page 22: Applying The Cuff
ENGLISH Do not move during measurement, it will have a delayed effect on the patient’s blood flow. The device need to be placed for 2 hours from the minimum storage temperature to being ready for its intended use. The device need to be placed for 4 hours from the highest storage temperature to being ready for its intended use. Note The following conditions may also cause changes in the blood pressure measurement value. - Page 23 ENGLISH 2. Stretch cuff into a barrel for the arm can conformable enter into the barrel. 3. Left arm penetrate through the cuff, the air tube of the cuff will pass the top of your palm. 4. Wrap the cuff to your upper arm. Make the air tube inside the forearm and aligned with your middle finger.
-
Page 24: Bp Measurement
ENGLISH 10.3 BP Measurement The user can be set to three different types(adult, pediatric and newborn). Set it through the [USER TYPE] option in [SYS- TEM SETUP] menu. Note When the patient is a newborn, please select the newborn mode and select the appropriate size of the cuff to measure, otherwise it may cause harm to the patient. -
Page 25: Chapter 11 Memory Function
ENGLISH Chapter 11 MEMORY FUNCTION The sphygmomanometer is designed to store the blood pressure, pulse rate values and the date and time when measured, which are up to 100 groups. If there have been stored 100 groups, the earliest results will be deleted when saving the 101 group of measurement results. -
Page 26: Chapter 12 Spo 2 Measurement Function
ENGLISH Chapter 12 MEASUREMENT FUNCTION (This chapter is only suitable for European Union market) Precautions during SpO Measurement: Note • Make sure the nail covers the light. The probe cable should be on the backside of the hand. Improper probe placement or improper contact with the test site will influence the measurement. - Page 27 ENGLISH • As the measure is taken on the basis of arteriole pulse, substantial pulsating blood flow of subject is required. For a sub- ject with weak pulse due to shock, low ambient/body temperature, major bleeding, or use of vascular contracting drug, the SpO waveform (PLETH) will decrease.
- Page 28 ENGLISH boxyhaemoglobin (COHb), methaemoglobin (MetHb) and sulfhaemoglobin (SuHb)), but the testee may appear hypoxia, it is recommended to perform further assessment according the clinical situations and symptoms. • Pulse oxygen has only reference significance for anemia and toxic hypoxia, as some patients with severe anemia still show better pulse oxygen measurements.
-
Page 29: Chapter 13 Spo 2 Measurement Method
ENGLISH • Please refer to related medical literature for detailed clinical restrictions and contraindications, • This device is not used for treatment purpose. • Do not use the SpO probe during MRI and CT scanning, as the induced current may cause burns. •... -
Page 30: Chapter 14 Installation Of The Software
ENGLISH • Intravenous dyestuff. • Excessive patient movement. • External light. • Improper SpO probe installation or incorrect contact position of the patient. • Temperature of SpO probe(optimal temperature range: 28°C ~ 40°C). • Place the SpO probe on an limb that has a blood pressure cuff, arterial catheter, or intravascular line. •... -
Page 31: Chapter 15 Error Message
ENGLISH follow install instructions below: Open Windows Explorer. Click on the root CD-ROM directory. Double click file setup software. Follow the instructions in the screen. Refer to “Software Help”for details about the operation method of the PC software. Chapter 15 ERROR MESSAGE Error message will be displayed in the screen if there is something wrong when measuring. -
Page 32: Chapter 16 Troubleshooting
ENGLISH Excessive movement The signal extent is too big owing to Over range the arm or body moving or other rea- Keep arm, body still, measure again Saturated signal sons when measuring Time out It takes too much time Chapter 16 TROUBLESHOOTING Abnormal Phenomenons Causes... - Page 33 ENGLISH Power off suddenly when inflating No use for a long time, the power of Replace all four batteries with new batteries can be exhausted owing to ones. the changed temperature Hold the on/off button but can not start Power of batteries can be exhausted Replace all four batteries with new the device ones.
-
Page 34: Chapter 17 Keys And Symbols
ENGLISH Chapter 17 KEYS AND SYMBOLS Symbol Description Symbol Description Follow instructions for use Interface for connecting cuff Systolic pressure Adult Mean pressure Pediatric Diastolic pressure Neonatal Pulse rate (bpm) INFO Information Open the Prompt sound indication Close the Prompt sound indication Low-power Full-power 1. -
Page 35: Chapter 18 Maintenance, Cleaning And Keeping
ENGLISH Manufacturer Date of manufacture Keep away from sunlight Keep in a cool, dry place Fragile, handle with care This side up 106kPa Atmospheric pressure limitation Moisture limitation 70kPa 55°C Caution: read instructions (warnings) Store between -2 and 55°C carefully -20°C WEEE disposal Chapter 18... - Page 36 ENGLISH Caution • High pressure disinfection to the device and accessories is not allowed. • Do not let water or cleaning agent flow into the socket to avoid device damage. • Do not soak the device and accessories in liquid. •...
-
Page 37: Chapter 19 Nibp Specification
ENGLISH • Aged cuff may result in inaccurate measurement, please replace the cuff periodically accord- ing to the user manual. • To avoid device damage, keep the device out the reach of children and pets. • Avoid the device close to extreme high temperature such as fireplace, otherwise the device performance may be affected. - Page 38 ENGLISH Overpressure protection adult mode 295±5 mmHg (39,33±0,67 kPa) pediatric mode 240±5 mmHg (32±0,67 kPa) neonatal mode 145±5 mmHg (19,33±0,67 kPa) Inflation adult 160±5 mmHg (21,33±0,67 kPa) pediatric 120±5 mmHg (16±0,67 kPa) neonatal 70±5 mmHg (9,33±0,67 kPa) Prompt Range adult mode SYS: 40~270 mmHg - DIA: 10~215 mmHg pediatric mode SYS: 40~200 mmHg - DIA: 10~150 mmHg...
- Page 39 ENGLISH Safety classification Class Ⅱ equipment (power supplied by power adapter)/ Internally powered equipment (power supplied by batteries) Type BF applied part Service life The service life of the device is five years or 10000 times of BP measurement. Date of manufacturer See the label Accessories Standard Configure:...
-
Page 40: Chapter 20 Spo 2 Specification
ENGLISH Chapter 20 SPECIFICATION (This chapter is only suitable for European Union market) Name Probe (Accessory Separate Sale) Measurement Range Measuring Range: 0%~100%; Pulse Rate Measuring Range: 30 bpm~250 bpm; Resolution 1bpm Measurement Accuracy 70%~100% ±2% 0%~69% undefined ±2 bpm or ±2% (select larger) Measurement Performance In Weak Filling Condition Pulse-filling ratio : 0.4% error... -
Page 41: Appendix
ENGLISH APPENDIX Guidance and manufacturer’s declaration – electromagnetic emissions- for all EQUIPMENT and SYSTEMS Guidance and manufacturer’s declaration – electromagnetic emission The device is intended for use in the electromagnetic environment specified below. The customer of the user of the device should assure that it is used in such and environment. - Page 42 ENGLISH Guidance and manufacturer’s declaration – electromagnetic immunity – for all EQUIPMENT and SYSTEMS Guidance and manufacturer’s declaration – electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment.
- Page 43 ENGLISH Guidance and manufacturer’s declaration – electromagnetic immunity – for EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING Guidance and manufacturer’s declaration – electromagnetic immunity The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that it is used in such an environment.
- Page 44 ENGLISH NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
- Page 45 ENGLISH Recommended separation distances between portable and mobile RF communications equipment and the EQUIP- MENT or SYSTEM – for EQUIPMENT or SYSTEM that are not LIFE-SUPPORTING Recommended separation distances between portable and mobile RF communications equipment and the de- vice The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled.
- Page 46 ENGLISH Warning • Active medical devices are subject to special EMC precautions and they must be installed and used in accordance with these guidelines. • Electromagnetic fields can affect the performance of the device, so other equipment used near the equipment must meet the appropriate EMC requirements.
- Page 47 ENGLISH for the release of human body static electricity to the ground or equipment frame or the use of a bracelet to connect the human body to the equipment or the ground before establishing the connection should be described. The following cable types must be used to ensure that they comply with interference radiation and immunity standards: Name Length (m) Power adapter cable...



Need help?
Do you have a question about the CONTEC08A and is the answer not in the manual?
Questions and answers