Summary of Contents for Tecnomed Italia Skudo
- Page 1 V.8.00 Aerosol barrier Installation, operation and maintenance manual CODE DP2003S...
-
Page 3: Table Of Contents
CONTENTS SECTION PAGE 1- SYMBOLS 1.1- WARNING LABELS 2- GENERAL WARNINGS 2.1 - GENERAL INSPECTION 2.2 - IN-TRANSIT DAMAGES FOR DELIVERIES IN ITALY 2.3 - IN-TRANSIT DAMAGES FOR DELIVERIES OUTSIDE ITALY 2.4 - TRANSPORT AND STORAGE CONDITIONS 3- SAFETY RULES FOR INSTALLATION 3.1 - WORK ENVIRONMENT 3.2 - MAXIMUM LOADS 4- SAFETY PROVISIONS... -
Page 4: 1- Symbols
1- SYMBOLS MANUFACTURER SYMBOL. This symbol is placed on the product next to the name and address of the manufacturer. SERIAL NUMBER SYMBOL. This symbol is placed on the product next to the serial number of the device. CE MARKING. This symbol indicates that the product has a CE marking in conformity with the provisions of Directive CEE 93/42 and subsequent amendments (Class I Devices). -
Page 5: Warning Labels
• All the materials contained in this manual are the property of Tecnomed Italia and/ or of the companies represented by it. The images are not binding and are given for explanatory purposes only. -
Page 6: General Inspection
6. Do not return the product to Tecnomed Italy srl before receiving an answer and an authorisation to do so. 7. Send the signed delivery note to Tecnomed Italia srl. 8. Leave the product and the packaging as they are. -
Page 7: In-Transit Damages For Deliveries Outside Italy
2.3 - IN-TRANSIT DAMAGES FOR DELIVERIES OUTSIDE ITALY ATTENTION! Tecnomed Italy srl shall not be liable for damages occurred during transit. Check the goods as soon as you receive them! If the package presents clear signs of damage upon delivery, proceed as follows: 1. -
Page 8: 3- Safety Rules For Installation
3- SAFETY RULES FOR INSTALLATION DANGER! Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot be held liable for personal injury and/or direct or indirect property damage deriving from failure to comply with the instructions in this manual and/or from incorrect installation and/or use of the device and its accessories and/or from improper and/or lack of cleaning and/or maintenance. -
Page 9: 4- Safety Provisions
ATTENTION! Do not expose the device to direct sunlight or to sources of UV light. ATTENTION! Disconnect the pneumatic, hydraulic and electrical power supply at the end of the day (if present). Tecnomed Italia s.r.l. shall not cover damages caused by failure to comply with the indications above. -
Page 10: Safety Requirements
BIOLOGICAL RISK - SKUDO™ blocks and sucks only the aerosols that follow the path from the patient's oral cavity to the operator's face.SKUDO™ cannot be classified as a personal protective equipment as it is not wearable and is not held in the hand by the op- erator.For this reason it is necessary to always wear PPE as per current regulations. -
Page 11: Standards/Certifications
5.2 - STANDARDS/CERTIFICATIONS Medical device The SKUDO™ device bears the CE mark. The SKUDO™ device is classifi ed as a class I medical device according to rule 1 of annex IX of Directive 93/42/EEC. The device also complies with the following standards:... -
Page 12: Dimensions
5.4 - DIMENSIONS 5.5 - PACKING LIST... - Page 13 PHOTO DESCRIPTION Collar for the head support arm SKUDO™ with arm Suction conveyor Suction adapters 17/11 mm Quick male fi tting - 4 mm tube Quick female fi tting - 1/8F Instruction manual M6 knob 2 M5 knobs with washers...
-
Page 14: Identification Plates
5.6 - IDENTIFICATION PLATES The plate is located on the headrest collar. Data on the plate • Name of the manufacturer. • Name of the device. • Product code. • Serial number. • Air pressure • Suction depression Guide to reading the serial number 1308001 Progressive number Year of manufacture... -
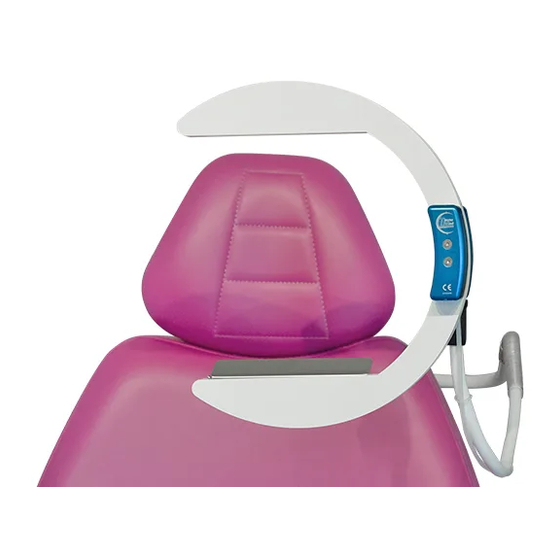
Page 15: Product Profile
5.7 - PRODUCT PROFILE Suction unit Toggle valve Compressed air outlet Micro suction input Suction output Pneumatic unit Flexible arm Adaptor Pneumatic tube 4 x 0.75 mm Headrest collar... -
Page 16: 6- Installation
6- INSTALLATION DANGER! Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot be held liable for personal injury and/or direct or indirect property damage deriving from failure to comply with the instructions in this manual and/or from incorrect installation and/ or use of the device and its accessories and/or from improper and/or lack of cleaning and/ or maintenance. -
Page 17: Supply Connection - Standard Installation
6.1 - SUPPLY CONNECTION - STANDARD INSTALLATION AIR: • Install the supplied female quick coupling on the water unit or on the back of the chair. • Connect it to a 4 bar pneumatic supply (NB: if necessary, install a pressure reducer - Cod.PV1803MR) •... -
Page 18: Supply Connection - Fixed Installation
Recommended when double suction is always used in the oral cavity. WARNING! If all the dental units of the surgery/practice work simultaneously using SKUDO™ and double suction cannula, it is necessary to check the flow rate of the suction system as it could be under sized. -
Page 19: 7- Instructions For Use
22-23. 8- POSITIONING The SKUDO™ head is keyed onto an arm with a fl exible end, which in turn is mounted on an arm that rotates 160°, thanks to the presence of 2 stops, quickly assuming the rest or working position. - Page 20 70° If necessary, it is possible Once in the working position, to block the SKUDO™ arm in intermedi- engage in the lower opening ate positions by acting the suction conveyor on the central knob. duly disinfected. WARNING! DANGER! Hold the SKUDO™ head in the centre Never handle SKUDO™...
-
Page 21: 9- Operation
9- OPERATION Insert adapter A on the surgical cannula and insert it into hole C. In the case of interventions with limited aerosols, it is also possible to use the saliva suction cannula using adapter B. 17 mm 11 mm Move the lever to release the air blade. -
Page 22: External Cleaning
10 - EXTERNAL CLEANING 1 - Remove the conveyor and disinfect it with a sprayer GREEN & CLEAN SK 2 - Clean the surfaces with GREEN & CLEAN MK WIPES. WARNING! Never spray directly on the surface.The disinfectant could penetrate the slits damaging the elasticity of the Plexiglas parts. -
Page 23: Internal Cleaning
11 - INTERNAL CLEANING 1 - With active suction on SKUDO™ brush the lower slit with a brush soaked in alcohol-free disinfectant. WARNING! Do not exert high pressure on the side walls of the slit, danger of breakage. 11.1 - RECOMMENDED DISINFECTANT GREEN &... -
Page 24: Maintenance
ATTENTION! The staff of the practice is responsible for making sure that the product is serviced in due time. Tecnomed Italia s.r.l. denies any liability, expressed or implied, and cannot be held liable for injuries to persons and/or direct or indirect damages to property, deriving from failure to perform the safety technical controls and maintenance operations. -
Page 25: Troubleshooting
* The resistance of the arm is guaranteed collapses for 5 years The collar is released from the headrest Insert the side nuts of the length rod when the Installation error suitable for a professional fixing SKUDO™ head is moved... -
Page 26: 14- Related Items
14- RELATED ITEMS To fi nd all items related to this product, visit: www.dentalastec.it 15- DISPOSAL This equipment complies with the European Directive 2002/96/CE on waste electrical and electronic equipment (WEEE). By ensuring this product is disposed of correctly, you will help prevent potential negative consequences to the environment and human health, which could otherwise arise from improper handling of the product at the end health, which could otherwise arise from improper handling of the product at the end... -
Page 27: Warranty
Disconnect the pneumatic, hydraulic and electrical power supply at the end of the day (if present). Tecnomed Italia srl shall not cover damages caused by failure to comply with the indications above. Not covered by warranty: •... -
Page 28: Declaration Of Conformity
17 - DECLARATION OF CONFORMITY EC DECLARATION OF CONFORMITY 93/42/EEC MODEL:SKUDO™ Tecnomed Italia Srl declares, under its own responsibility, that the Class I medical device described below Model: SKUDO™ Code:DP2003S Progressive number:__________________ to which this declaration refers: complies with the Essential Requirements (annex 1) and the provisions of the Directives... -
Page 29: 18- Certificate Of Warranty
Timbro del rivenditore-Dealer’s stamp Cachet d’achat-Sello del revendedor Data d’acquisto-Purchase date Date d’achat-Fecha de compra Tecnomed Italia s.r.l. Via Salvador Allende n.2, 61040 Castelvecchio di Monte Porzio (PU) Italy Phone +39 0721 95 51 25 Fax +39 0721 95 52 29 - www.dentalastec.it... -
Page 30: Pneumatic Test
19 - PNEUMATIC TEST Product label Operator code Decibels within the norm No internal air leak No loss of internal depression 4 bar air curtain effective Pass... - Page 32 Tecnomed Italia s.r.l. Via Salvador Allende n.2, 61040 Castelvecchio di Monte Porzio (PU) Italy Phone +39 0721 95 51 25 Fax +39 0721 95 52 29 - www.dentalastec.it...


Need help?
Do you have a question about the Skudo and is the answer not in the manual?
Questions and answers