Table of Contents
Advertisement
Available languages
Available languages
Carpentier-Edwards PERIMOUNT Magna
• Pericardial Bioprosthesis Model 3000TFX Aortic
• Bioprótesis pericárdica modelo 3000TFX aórtica
• Prótese Biológica Pericárdica Modelo 3000TFX Aórtico
DIRECTORY
English . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Español . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Português . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
References / Bibliografía / Bibliografia . . . . . .26– 27
Specifications / Especificaciones /
Especificações . . . . . . . . . . . . . . . . . . . . . . . . . 28– 32
Figures / Figuras / Figuras . . . . . . . . . . . . . . . 33–34
Symbol Legend / Significado de los Símbolos /
Legenda dos Símbolos . . . . . . . . . . . . . . . . . . . . . .35
English
Instructions for Use
1. Device Description
The Carpentier-Edwards PERIMOUNT Magna pericardial
bioprosthesis (also referred to as the Magna bioprosthesis) is a
trileaflet valve comprised of bovine pericardium that has been
preserved in a buffered glutaraldehyde solution and mounted
on a flexible frame.
The tissue is fixed using the novel Neutralogic fixation process
in which the tissue is placed in a stress-free bath of
glutaraldehyde solution.
The bioprosthesis is treated according to the Edwards ThermaFix
process, which involves heat treatment of the tissue with
glutaraldehyde and uses ethanol and polysorbate-80 (a surfactant).
The bioprosthesis is packaged and terminally sterilized in
glutaraldehyde. Glutaraldehyde is shown to both reduce the
antigenicity of tissue Xenograft valves and increase tissue stability
(Refs. 10 & 12). Glutaraldehyde alone has not been shown to affect
or reduce the calcification rate of the valve.
Edwards Lifesciences, the stylized E logo, Edwards,
Carpentier–Edwards, ThermaFix, Neutralogic, PERIMOUNT and
PERIMOUNT Magna are trademarks of Edwards Lifesciences
Corporation; Carpentier–Edwards and PERIMOUNT are
registered in the U.S. Patent and Trademark Office.
Elgiloy is a trademark of Elgiloy Limited Partnership.
Model 3000TFX PERIMOUNT Magna Aortic
Modelo 3000TFX PERIMOUNT Magna aórtica
Modelo 3000TFX PERIMOUNT Magna Aórtico
The frame is designed to be compliant at the orifice as well as at
the commissures. The compliance of the commissure supports
is intended to reduce the loading shock at the valve
commissures and free margin of the leaflets (Ref. 42). The
compliance of the orifice is intended to reduce the stress on the
leaflet. The compliant orifice concept is based on the physiology
and mechanics of natural heart valves and reported experience
with implantation of unstented homografts (Refs. 5 & 7).
The lightweight wireform frame is made of Elgiloy, a corrosion-
resistant alloy, chosen because of its superior spring efficiency
and fatigue resistant characteristics, and is covered with a
knitted polyester fabric.
A thin Elgiloy/polyester film laminate band surrounds the base of
the wireform frame providing structural support for the orifice.
To this frame is attached a soft, silicone-rubber suture ring that
is covered with a porous, seamless polytetrafluoroethylene cloth
to facilitate tissue ingrowth and encapsulation. The aortic sewing
ring has been scalloped to conform to the natural aortic root. The
compliant nature of the suture ring facilitates coaptation between
the valve and an often irregular or calcific tissue bed.
An integral valve holder is attached to the valve by means of
sutures to facilitate handling and suturing the valve during
implantation. The holder is easily detached by the surgeon
(see 11.2 Handling and Preparation Instructions).
The sewing ring diameter on the Carpentier-Edwards
PERIMOUNT Magna pericardial bioprosthesis has been reduced
to facilitate implantation in patients with small aortic roots.
1
CP1036-20
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Edwards Lifesciences 3000TFX PERIMOUNT Magna Aortic
-
Page 1: Instructions For Use
The holder is easily detached by the surgeon (see 11.2 Handling and Preparation Instructions). The sewing ring diameter on the Carpentier-Edwards Edwards Lifesciences, the stylized E logo, Edwards, PERIMOUNT Magna pericardial bioprosthesis has been reduced Carpentier–Edwards, ThermaFix, Neutralogic, PERIMOUNT and to facilitate implantation in patients with small aortic roots. -
Page 2: Indications For Use
2. Indications for Use DO NOT EXPOSE the valve to any solutions, chemicals, antibiotics, etc. except for the storage solution or sterile Pericardial valves are indicated for use in patients suffering physiological saline solution, as irreparable damage to the leaflet from valvular heart disease. -
Page 3: Adverse Events
This tag should not be detached from the valve Material Safety Data Sheet available from Edwards Lifesciences. until implant is imminent. Care should be exercised to avoid cutting or tearing the suture ring cloth during removal. -
Page 4: Clinical Studies
diatheses related to the use of anticoagulant therapy, and It is possible that these complications could lead to: malfunctions of the valve due to distortion at implant, fracture • Reoperation of the Elgiloy wireform, or physical or chemical deterioration of valve components. -
Page 5: Individualization Of Treatment
This data was compiled as of July 1994 from a multi-center bioprosthesis is provided sterile and nonpyrogenic packaged in clinical trial conducted by Edwards Lifesciences. Follow-up on glutaraldehyde, in a plastic jar to which a seal has been applied. this post-approval cohort is continuing, and periodic updates... -
Page 6: Directions For Use
Contact the local Using gloved hand, attach the handle to the valve holder while supplier or representative of Edwards Lifesciences to make the valve is still in the container. To do this, simply insert the arrangements for return, authorization, and replacement. - Page 7 Supra-annular sizing and implantation: Handle/Holder Removal Using supra-annular technique, the sewing ring of the valve The integral holder and attached handle are removed as a unit is placed above the annulus, maximizing valve orifice area. at the completion of the suturing procedure in the following A larger valve size can often be implanted using a supra- manner (see Figure 3): annular technique compared to an intra-annular technique.
-
Page 8: Patient Information
11.5 Accessory Sterilization 12. Patient Information The Model 1111 handle and the Model 1130 sizers are supplied 12.1 Registration Information nonsterile and must be sterilized before using. The handles and An Implantation Data Card is included in each device package sizers must be cleaned and resterilized prior to each use. - Page 9 2. Indicaciones de uso Español Las válvulas pericárdicas están indicadas para su uso en pacientes con valvulopatías. Una valvulopatía aórtica es una enfermedad en la Instrucciones de uso que se dan una o varias de las siguientes condiciones: obstrucción o estenosis de la válvula cardíaca aórtica; fugas en la válvula 1.
- Page 10 Lifesciences. Sólo se deben usar los calibradores de diagnosticar y tratar adecuadamente cualquier complicación Edwards Lifesciences para seleccionar el tamaño de la relacionada con la válvula, especialmente las relacionadas con válvula; la selección de la válvula puede no ser la adecuada fallos del material.
-
Page 11: Efectos Adversos
• Debido a la relativa flexibilidad del marco, éste debe contengan calcio en el postoperatorio, así como la ingestión manipularse con sumo cuidado para evitar la formación de excesiva de leche y derivados lácteos en los niños. Los pliegues o deformaciones del stent que pueden provocar estudios realizados en animales (ref. - Page 12 6.2 Efectos adversos potenciales Entre la población afectada por DVR, había un total de 24 (34,3%) hombres y 46 (65,7%) mujeres con una edad media de 62,9 Los efectos adversos que se pueden asociar al empleo de las (±12,7) años (± desviación estándar) y con edades comprendidas válvulas cardíacas protésicas son: entre 31 y 94 años.
-
Page 13: Individualización Del Tratamiento
La bioprótesis pericárdica PERIMOUNT Magna, modelo Departamento de marketing de cirugía cardiovascular de 3000TFX se proporciona estéril y apirógena en una solución de Edwards Lifesciences LLC, One Edwards Way, Irvine, glutaraldehído, dentro de un frasco de plástico sellado. CA 92614-5686, EE.UU. - Page 14 Póngase en contacto con el proveedor o el representante local de Edwards Lifesciences para acordar la devolución, Retire el sello y desenrosque la tapa del frasco. El frasco debe autorización y sustitución.
- Page 15 11.3 Dispositivo de implantación Para realizar la calibración correctamente, el calibrador debe estar paralelo al plano del annulus, y todo el Debido a la complejidad y diversidad en el procedimiento calibrador, incluyendo la parte simulada del anillo de sutura, quirúrgico de reemplazo de válvulas cardíacas, se deja a elección debe pasar a través del annulus (Figura 6a-6c).
- Page 16 Cuando se desecha una válvula o deben limpiar y volver a esterilizar antes de cada uso. Los se sustituye un dispositivo de Edwards Lifesciences, se debe calibradores se deben examinar para ver si presentan signos de comunicar el dato al registro de pacientes con implantes.
- Page 17 2. Indicações de Utilização Português As válvulas pericárdicas estão indicadas para utilização em doentes que sofram de doença valvular cardíaca. A doença Instruções de Utilização valvular cardíaca de origem aórtica é uma situação que envolve qualquer uma das seguintes condições: obstrução ou estenose da 1.
- Page 18 O doente candidato à substituição valvular deve ser glutaraldeído, consultar a Folha dos Dados de Segurança do completamente informado, antes da cirurgia, sobre os riscos e Material disponível junto da Edwards Lifesciences. benefícios da mesma. • A prótese biológica Magna possui uma configuração exclusiva Nota: As próteses biológicas deverão ser utilizadas com...
- Page 19 6.1 Reacções Adversas Observadas distorção do orifício. A Edwards Lifesciences recebeu alguns À semelhança do que se verifica com todas as próteses valvulares relatórios nos quais a utilização de suturas de colchoeiro cardíacas, algumas reacções adversas graves, por vezes mesmo...
- Page 20 Nota: Com base nos relatórios presentes na literatura sobre 7. Estudos Clínicos válvulas tecidulares (Refs. 3, 18, 23, 26, 36, 48, 49 e 54), parece Grupo de Doentes Pré-Aprovação existir um aumento na incidência de calcificação dos folhetos em doentes com idade inferior a 20 anos. Neste domínio, estudos Os dados clínicos, disponíveis para 719 doentes necessitando de efectuados em animais (Ref.
-
Page 21: Individualização Do Tratamento
Edwards Lifesciences. O acompanhamento deste grupo pós- endocardite/sépsis e 13 eventos deveram-se a disfunção valvular. aprovação continua, e serão disponibilizadas actualizações A taxa actuarial sem explante é... -
Page 22: Indicações De Utilização
9. Informações de Aconselhamento para os Doentes 10.3 Conservação As próteses biológicas da Carpentier-Edwards deverão ser Aconselha-se um acompanhamento médico cuidadoso e conservadas entre 10°C e 25°C (50-77°F). Recomenda-se uma permanente (no mínimo, uma consulta por ano), para que seja inspecção e rotação do stock a intervalos regulares, para possível diagnosticar e tratar adequadamente quaisquer garantir que as válvulas são utilizadas antes do prazo de... - Page 23 Com a mão protegida por luva, encaixar o punho no suporte da 3. Medir o tamanho do annulus utilizando exclusivamente os válvula enquanto a válvula se encontra ainda no recipiente. medidores da Carpentier-Edwards, Modelo 1130 aórtico Para o fazer, basta introduzir o punho no suporte da válvula e (Figuras 4a-4c).
- Page 24 Precaução: Em virtude da temperatura intensa e das Suporte e Punho da Válvula condições de iluminação presentes no campo operatório, a O conjunto punho/suporte é constituído por dois componentes: prótese biológica deverá ser irrigada com frequência uma peça descartável integrante, que é montada fisicamente na (recomenda-se de 1 em 1 ou de 2 em 2 minutos) em ambas válvula pelo fabricante, e um punho maleável (Modelo 1111 as faces com soro fisiológico esterilizado, para manter a...
- Page 25 11.6 Devolução das Próteses Biológicas Explantadas 12.2 Manual do Doente A Edwards está extremamente interessada em obter amostras Os materiais de informação para os doentes podem ser obtidos clínicas recuperadas de próteses biológicas da Carpentier- junto da Edwards ou do especialista clínico de vendas da Edwards. Edwards para análise.
- Page 26 References / Bibliografía / Bibliografia 1. American Heart Association. Guide to Anticoagulant Therapy 17. Ferrans, V.J., et al. Structural Changes in Glutaraldehyde- Part 1: Heparin and Part 2: Oral Anticoagulants. Circulation, Treated Porcine Heterografts Used as Substitute Cardiac 89(3): 1449-1480, 1994. Valves.
- Page 27 33. Levine, F.H., et al. Hemodynamic Evaluation of Hancock and 49. Reul, G.J., et al. Valve Failure with the lonescu-Shiley Bovine Carpentier-Edwards Bioprostheses. Circulation, Pericardial Bioprosthesis: Analysis of 2680 Patients. 64(Suppl. II):192-195, 1981. J. Vasc. Surg., 2(1):192-204, 1985. 34. Liao, K., et al. Bovine Pericardium Versus Porcine Aortic Valve: 50.
- Page 28 Table A. Nominal Specifications (mm) Carpentier-Edwards PERIMOUNT Magna Pericardial Bioprothesis, Model 3000TFX Size 19 mm 21 mm 23 mm 25 mm 27 mm 29 mm A. Stent Diameter (Wire Form) B. Internal Diameter (Stent I.D.) C. Profile Height D. External Sewing Ring Diameter CP1036-21 Note: For sizing, see surgical procedure recommendations.
- Page 29 Table 1. Summary of Complication Rates, Model 2700 Isolated AVR Population DVR Population Post- % Event-Free Post- % Event-Free Operative operative at Six Years Operative operative at Six Years Complication % of Pts. % Per Pt.Yr. (Standard Error) % of Pts. % Per Pt.
- Page 30 Table 2. Postoperative Echocardiography Results, Model 2700 Valve Size 19 mm 21 mm 23 mm 25 mm 27 mm 29 mm Total Total N Avg. Months Postoperative 28.6 ± 7.2 34.9 ± 8.6 36.9 ± 9.2 39.9 ± 7.6 31.4 ± 15.9 15.3 ±...
- Page 31 Table 3. Effectiveness Outcomes, Functional NYHA, Model 2700 Preoperative Postoperative NYHA Functional Class NYHA Functional Class Expiration Available Not Available Tabla 3. Criterios de valoración de eficacia, grados funcionales de la NYHA, modelo 2700 Preoperatorio Postoperatorio Clase funcional NYHA Clase funcional NYHA Expiración disponible No disponible...
- Page 32 Table 4. Summary of all Valve-Related Complication Rates (N = 267), Model 2700 Operative Period Postoperative Period (≤30 Days) (>30 Days) % of Pts. % Per Pt. Year Complication No. of Incidences No. of Incidences Thromboembolism/Thrombus 1.45 Endocarditis 0.33 Valve Dysfunction 0.37 1.60 Perivalvular Leak...
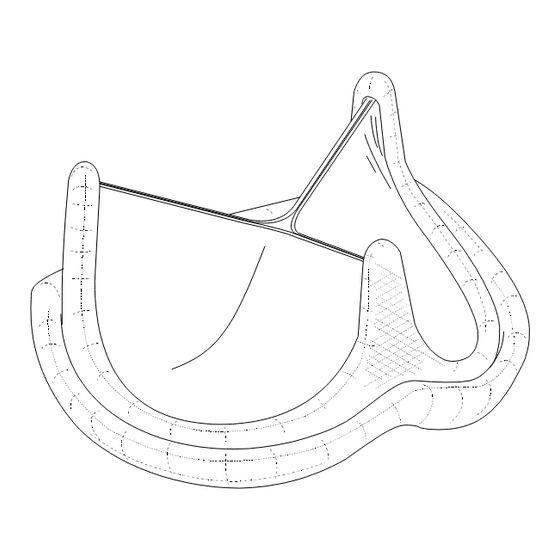
- Page 33 CP1036-46 Figure 1 Aortic / Figura 1 Aórtica / Figura 1 Aórtico CP1036-47 CP1036-48 Figure 2 Aortic / Figura 2 Aórtica / Figura 2 Aórtico CP1036-45 CP1036-33 Figure 4a Aortic Sizer / Figura 4a Calibrador aórtico Figure 3 Aortic / Figura 3 Aórtica / Figura 3 Aórtico Figura 4a Medidor Aórtico...
- Page 34 CP1036-34 CP1036-35 CP1036-36 Figure 5a Supra-Annular Measurement Figure 4b Cylindrical Sizer Figure 4c Replica Sizer Figura 5a Calibración supra-anular Figura 4b Calibrador cilíndrico Figura 4c Calibrador réplica Figura 5a Medição Supra-Anular Figura 4b Medidor Cilíndrico Figura 4c Medidor Réplica CP1036-37 CP1036-38 CP1036-29 Figure 5c Supra-Annular Measurement...
- Page 35 Symbol Legend • Légende des symboles • Zeichenerklärung • Significado de los Símbolos • Legenda dei simboli • Lijst van symbolen • Symbol tekst • Förklaring till symbolen • Legenda dos Símbolos English Français Deutsch Español Italiano Nederlands Dansk Svenska Português Catalogue Numéro de...
- Page 36 01/05 Edwards Lifesciences Services GmbH 149256001 Rev. A Edisonstr. 6 © Copyright 2005, Edwards Lifesciences LLC D-85716 Unterschleissheim, Germany All rights reserved Edwards Lifesciences LLC One Edwards Way Irvine, CA 92614-5686 USA 149256001A...

Need help?
Do you have a question about the 3000TFX PERIMOUNT Magna Aortic and is the answer not in the manual?
Questions and answers