Table of Contents
Advertisement
Quick Links
Via Artigianale Croce n.13 - 42035 Castelnovo ne' Monti (RE) - ITALY
Tel. +39.0522.612080 - Tel./Fax +39.0522.729796
www.mantisitalia.it - Resp.Tecnico: tecnico@mantisitalia.it
Use and Maintenance Instructions
Integrated treatment system with endodermic massage
and application of pulsed magnetic fields with stochastic resonance
MR991
The manual must be kept in a safe place for the entire life of the machine. Please read carefully the
manual before operating any part of the equipment
Advertisement
Table of Contents

Summary of Contents for Mantis MR991
- Page 1 Integrated treatment system with endodermic massage and application of pulsed magnetic fields with stochastic resonance MR991 The manual must be kept in a safe place for the entire life of the machine. Please read carefully the manual before operating any part of the equipment...
-
Page 2: Table Of Contents
INDEX DECLARATION OF CONFORMITY CE ................ 4 GENERAL INFORMATION ................. 5 1.1 Equipment description..................5 1.2 Definitions and symbols used in the manual ..........5 SCOPE........................6 2.1 Intended use....................6 2.2 Reasonably foreseeable misuse ..............6 TECHNICAL DATA ....................8 EQUIPMENT DESCRIPTION ................ - Page 3 ACCESSORIES AND OPTIONAL ITEMS ............30 Accessories ..................... 30 Installation of accessories ................ 30 9.2.1 Handpiece without PMF system – Code MN50043 ......... 30 EQUIPMENT DECOMISSIONING ..............31 PROBLEMS AND SOLUTIONS ................ 31 SPARE PARTS....................32 GENERAL WARRANTY TERMS AND CONDITIONS ........33...
-
Page 4: Declaration Of Conformity Ce
Electromedical equipment Part 1: General requirements for basic safety and essential performance The manufacturer also declares that the technical construction file is kept at: MANTIS S.R.L. Via Artigianale Croce 13 - 42035 Castelnovo ne’ Monti (RE) - Italy Castelnovo ne’ Monti (RE), .. -
Page 5: General Information
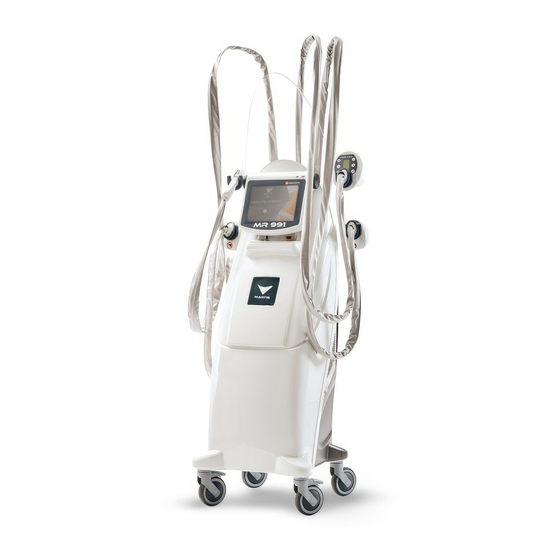
GENERAL INFORMATION 1.1 Equipment description MR991 is a machine intended for beauty operators and designed to improve body and face appearance. The MR991 system combines two technologies which can be easily programmed by the operator: - the MRM (mechanical manipulation with motorised rollers) which carries out an endodermic massage thanks to the mechanical movement of rollers and the microsuction. -
Page 6: Scope
SCOPE 2.1 Intended use MR991 is designed for face and body beauty treatment which is carried out with special handpieces driven by a software that also guides the operator throughout the entire treatment. A “checkup” form on the Customer’s general state of health is previously filled in to evaluate any risk factors which could lead to the exclusion of some treatment phases. - Page 7 With the exception of the above-mentioned cases, in the following cases MR991 (in any of its functions) must be used carefully and only with the doctor’s approval: - Pregnancy - Asthma - Tumours (neoplasias) - Scleropathy - Lymphopathy - Mesotherapy...
-
Page 8: Technical Data
TECHNICAL DATA MR 991 Width (W) 600 mm (23.62 in) Depth (D) 600 mm (23.62 in) Height (H) 1,950 mm (76.77 in) Weight 110 Kg (242 lbs) Minimum working space required around the 900 mm (35.43 in) equipment Power cable length 2.5 m (8.2 ft) Working length of handpiece tubes 2 m (6.5 ft) -
Page 9: Equipment Description
EQUIPMENT DESCRIPTION 4.1 Equipment Touch-screen control panel Vixo handpiece Maxi DES handpiece Mini DES handpiece Maxenergy handpiece Key switch Emergency stop USB port Wheels 10 Wheels with brake system 11 Carrying handles... - Page 10 Vixo handpiece Maxi DES handpiece Mini DES handpiece Maxenergy handpiece 21 Transparent handpiece holder 22 Cable conduit cover 23 Handpiece connector cover 24 Ethernet connection 25 Main switch 26 Maxenergy connection 27 Electric power supply 28 Mini DES / Maxi DES connections 29 Mini DES / Maxi DES connections 30 Vixo connections 31 Filters...
-
Page 11: Handpieces
4.2 Handpieces A Mini DES handpiece A1 Start / Pause B Maxi DES handpiece B1 Start / Pause B2 Higher function level B3 Backwards movement of rollers B4 Scrolling menu functions B5 Lower function level B6 Function display C Vixo handpiece C1 Start / Pause D Maxenergy handpiece D1 Start / Pause... -
Page 12: General Safety Precautions
GENERAL SAFETY PRECAUTIONS Instructions Before carrying out any operation on the equipment, please read this instruction manual carefully in all its parts. The operator must be able to use the equipment in safety, have a suitable psychological and physical profile, not be under the effect of drugs or alcohol and be fully instructed on how the machine works. -
Page 13: Safety Devices
On the equipment, near the main switch and on the handpiece holder, you will find some pictograms displaying the safety requirements, as well as the CE marking. If the stickers become illegible they must be replaced. Apparecchio per trattamenti estetici Classe: 1 MR991 modello: Tipo: BF matricola ____________________ 230 Volt ~... -
Page 14: Description Of The Package And Storage
5) Take out packages A and B. 6) Remove protecting film C without using any sharp objects. 7) Unscrew the two blocking knobs D at the base of MR991. 8) Check packages A and B, which must contain: 1 transparent handpiece holder... -
Page 15: Transport And Installation
6.3 Transport Before moving the equipment, always check that it is stable and that the working area is free from obstacles, such as objects, animals or people. The equipment must be carried by two people having a suitable physical profile to be able to lift it (see TECHNICAL DATA). -
Page 16: Assembly
6.4.2 Assembly Carry out all assembly operations before connecting the equipment to the power supply. 1) Remove the cable conduit cover (22) by unscrewing the knob screw (22P). 2) Unscrew the four screws on the frame underneath the cable conduit cover which was previously disassembled. -
Page 17: Power Connection
9) Insert the end of the rod in the appropriate place (38). 10) Fix the case by using the clamp on the cable conduit cover (44). 11) Mount the connector and the suction hose in the appropriate places (34) and (37). Insert the hose by applying some pressure in order to ensure that it works properly. -
Page 18: Connection For Telecare (If Available)
6.4.4 Connection for telecare (if available) MR991 is provided with a telecare system which enables, in case of problems or malfunctions, to get a quick answer from the Technical Assistance Staff, thereby considerably reducing any "downtime" periods of the equipment. -
Page 19: Commissioning
COMMISSIONING 7.1 Equipment functions MR991 is equipped with four handpieces (see also 4.2 Handpieces) to treat different body areas using various techniques. 7.1.1 Maxi DES handpiece The biggest handpiece is made up of rollers moving in two directions, a pulsed suction system and a CMPS system. This handpiece is designed to treat the larger body areas such as buttocks and thighs. - Page 20 2) Adjust the angle of the touch-screen control panel (1) and secure it using the side knobs. 3) Switch on the control panel using the key switch (6). There are two keys included. Keep the spare key in a safe place (in case of loss you can request a new copy by quoting the code written under chapter 12 - Spare Parts).
- Page 21 6) Should you need to change the menu reference language, select “Language” from menu (C), otherwise skip to step 8. 7) From menu (D), select the appropriate language and click "Forward". 8) From menu (C), select "Date and Time" access corresponding settings (E) and use the arrows to adjust the parameters.
-
Page 22: Start A Treatment Cycle
Background information Before starting a treatment cycle, it is essential to fully inform the customer about any contraindication to treatments (or parts of them) carried out with the Mantis DES Technology of MR991. Invite the customer to fill in, in all its parts, the personal case history report provided (“checkup"), without omitting any important information which could be useful to evaluate the appropriate treatment. - Page 23 It enables to store specific treatments for each patient on a USB stick supplied with the machine. If the USB stick is not inserted, the function is disabled. 3) By selecting DES Technology on menu (F), it is possible to enter the list of default treatments which have been selected according to the most common...
- Page 24 5) Menu (L) is accessible from menu (F) by selecting the item “Maxenergy”. In this case, for the application of "Mantis Magnetic Cream", only the use of the Maxenergy handpiece with PMFs is required. From this menu, the treatment duration can be modified by using the buttons "+"...
- Page 25 8) At the end of the first phase (step 1) you will be explicitly asked whether or not you want to go to the next phase (step 2). If required, confirm and start the second treatment phase for the application cream.
-
Page 26: Function Control Buttons On The Handpieces (See 4.2 Handpieces)
7.3.3 Function control buttons on the handpieces (see 4.2 Handpieces) Maxi DES handpiece Maxi DES is provided with various control buttons interacting with those on the touch screen. This handpiece includes a small monitor where you can scroll the various function menus and change their settings. The control buttons also allow you to reverse the direction of the handpiece rollers during the treatment. -
Page 27: Repeat A Treatment Cycle
If a treatment has been interrupted, set its remaining duration time. 7.8 Recovery from power failure After a power failure, MR991 will switch on automatically, restarting from the initial self-test page. If a treatment has been interrupted, set its remaining duration time. -
Page 28: Equipment Maintenance
Routine maintenance MR991 is programmed to monitor and report on certain problems associated with maintenance, such as the need to clean the filters, etc. However regular routine activities are also required. At each treatment cycle Clean and disinfect the handpieces. -
Page 29: Vacuum Filters Cleaning And Cartridge Replacement
8.1.3 Vacuum filters cleaning and cartridge replacement For the correct functioning of the equipment, it is essential to carry out the filter cleaning and maintenance. Filters are located at the back of the equipment, behind the handpiece connector cover (23). 1) Remove the magnetic cover (23) by pulling gently. -
Page 30: Accessories And Optional Items
ACCESSORIES AND OPTIONAL ITEMS 9.1 Accessories: Description Code Body stocking (pack of 10 pieces)..............TN20010 Multisystem cream (face cream to be used with the machine and after the treatment) ............CM30021 Activer cream (body cream to be used with the machine and after the treatment) ............CA40032 Handpiece without PMF system ................MN50043 Switch key (2 pieces) .................. -
Page 31: Equipment Decomissioning
EQUIPMENT DECOMISSIONING Before dismantling and scrapping the equipment, disconnect the power supply by unplugging the machine. The equipment decommissioning process must be performed by qualified personnel with appropriate PPE (personal protective equipment) as laid down by current regulations. The equipment contains electronic components, electromechanical components, plastic and metal parts. -
Page 32: Spare Parts
SPARE PARTS Position Description Code Filters ...................... FT10010 Handpiece tabs ..................LM11020 Switch keys ..................... 032 482 Mini DES handpiece ................. MP13040 Maxi DES handpiece ................MP13041 Maxenergy handpiece ................MX16030 Vixo handpiece..................VX17020 Power cable ..................... CA14050 Tab removal tool ..................CM10723... -
Page 33: General Warranty Terms And Conditions
- shipments received with unsuitable or inappropriate outside package, which have been damaged during transport. If the product sent to Mantis s.r.l. has been somehow tampered with or if the labels indicating the serial number have been removed or damaged, Mantis s.r.l. reserves the right to assess the validity of the Warranty.

Need help?
Do you have a question about the MR991 and is the answer not in the manual?
Questions and answers