Table of Contents
Advertisement
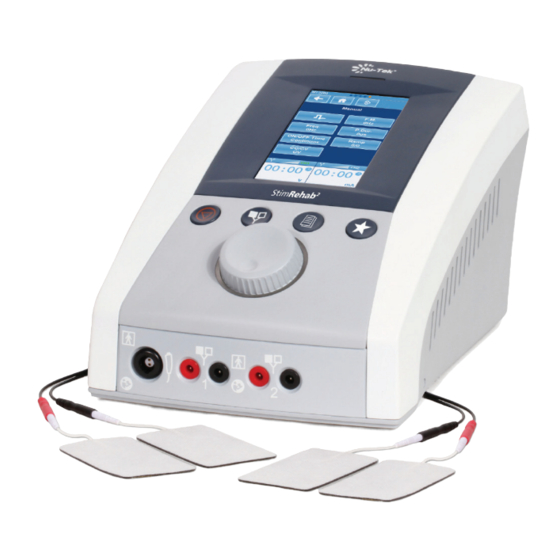
Nu-Tek® Stim Rehab2 − MT2200
Two Channel Electrotherapy
Features & Benefits:
• Provided with the most common currents: 4-pole IF, 2-pole
IF (Premode), NMS,Russian, Biphasic (TENS), Hi-Voltage
(HV), Microcurrent (MCR), DC: Galvanic, Faradic,
Diadynamic (DF, MF, CP, CP-ISO, LP, RS)
• Touch screen for easy operation
• 2-channel simultaneous monitoring
• 42 pre-set programs and 80 custom programs provided
Screen Shot:
physiosupplies.co.uk
The Warehouse Beck Bank West, Pinchbeck, Spalding, Lincolnshire, PE11 3QN
Power Supply:
Number of Channels:
IF Carrier Frequency:
Frequency:
Current Amplitude (peak):
Dimensions:
Weight:
Certification:
Safety Tests:
Electrical Safety Class:
NUPOWERCABLE
NUELECRUB6090
NUELECRUB70110 Rubber Electrodes - 70 x 110mm (Pair)
NUSPOENV70100
NUSPOENV80120
ACF35050
ACF35090
NUSTRAP751200
NUSTRAP75600
NULEADSTIMGY
NULEADSTIMBU
Optional Accessories:
Optional
Nu-Tek Trolley
NUTROLLEY
NUBATTERY
Code:
NUSTIM
Interferential Unit
AC100-240V 47/63 Hz
2 independently controllable
2 - 10 kHz
IF 1-200 Hz, Biphasic/NMS 1-250 Hz, HV
1-120 Hz, MCR 0.1-1000 Hz
IF Russian 100mA, Biphasic/NMS 200mA
HV 500 V, MCR 1000uA, DC 80mA
285mm (L) x 197mm (W) x 153mm (H)
2kg
FDA Clearance, EU MDD, CFDA, TGA, CMD-
CAS, Russian approved
IEC 60601-1, IEC 60601 2-5, IEC 60601-2-10
Class I, Type BF
Nu-Tek Power Cable
User Manual
Rubber Electrodes - 60 x 90mm (Pair)
Envelope Sponges - 70 x 100mm (Pair)
Envelope Sponges - 80 x 120mm (Pair)
AllCare Electrodes - 50 x 50mm Square (x4)
- Self Adhesive
AllCare Electrodes - 50 x 90mm Rectangle (x4)
- Self Adhesive
Nu-Tek Straps - 75 x 1200mm
Nu-Tek Straps - 75 x 600mm
Nu-Tek Stim Leads - Grey
Nu-Tek Stim Leads - Blue
Nu-Tek Trolley
Nu-Tek Battery Pack
Description:
Nu-Tek® Stim Rehab2 - MT2200
REDEFINING
| PHYSIOTHERAPY | FITNESS | MEDICAL
Phone: 01775 640972 Email: sales@physiosupplies.co.uk
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Nu-Tek Rehab Series
- Page 1 AllCare Electrodes - 50 x 50mm Square (x4) - Self Adhesive ACF35090 AllCare Electrodes - 50 x 90mm Rectangle (x4) - Self Adhesive NUSTRAP751200 Nu-Tek Straps - 75 x 1200mm NUSTRAP75600 Nu-Tek Straps - 75 x 600mm NULEADSTIMGY Nu-Tek Stim Leads - Grey NULEADSTIMBU...
- Page 2 Rehab – Series USER MANUAL 0197 www.nutekmedical.com...
- Page 3 Rehab-Series Rehab-Series Declaration of conformity: Shenzhen Dongdixin Technology Co.,LTD.declares that the ComboRehab-Series, StimRehab-Series and UltraRehab-Series complies with following normative documents: IEC60601-1,IEC60601-1-2,IEC60601-2-10,IEC60601-2-5,ISO7010 IEC61689,ISO14971,ISO10993-1,ISO10993-5,ISO10993-10 Complies with MDD 93/42/EEC and Amended by directive 2007/47/EC requirements...
-
Page 4: Product Description
Rehab-Series 1. Foreword 1.1 Intended User/Operator This manual has been written for the users of the Rehab-Series include ComboRehab- Series, StimRehab- Series and UltraRehab-Series. It contains general information on the operation, precautionary practices,and maintenance information. In order to maximize its use, efficiency,and the life of the system, please read this manual thoroughly and become familiar with the controls, as well as the accessories before operating the system. - Page 5 Rehab-Series StimRehab The StimRehab is equipped with two or four completely identical electrotherapy channels. The electrotherapy channels can be used in combination (linked) or totally independent. A comprehensive set of current waveforms is available, targeting both pain management and muscle stimulation applications. Protocols can run on linked or independent channels.
- Page 6 Rehab-Series module is designed for use with the StimRehab or ComboRehab only. EMG200 The EMG200 is designed for use with the StimRehab and the ComboRehab. With EMG electrodes the EMG200 generates the sEMG (Surface Electromyography) , ETS (Surface Electromyography with Triggered Stimulation) through which the EMG electrodes are attached to the patient.
- Page 7 Rehab-Series Vacuum module EMG module Battery module Name Model Mainframe (BTM200) (VAM200) (EMG200) CT2204 Mainframe (two channels electrotherapy, ComboRehab² VB ultrasound and combo+vacuum CT2200 module +EMG module) CT2205 Mainframe (two channels electrotherapy, ComboRehab² Vac CT2200 Plus ultrasound andcombo+vacuum module+ battery module) CT2206 (two channels electrotherapy, Mainframe...
- Page 8 Rehab-Series Vacuum module EMG module Battery module Name Model Mainframe (BTM200) (VAM200) (EMG200) MT2201 Mainframe Stim Rehab² Vac (two channels electrotherapy MT2200 +vacuum module) MT2202 Mainframe (two channels electrotherapy Stim Rehab² Bio MT2200 +EMG module) MT2203 Mainframe (two channels electrotherapy Stim Rehab²...
- Page 9 Rehab-Series Vacuum module EMG module Battery module Name Model Mainframe (BTM200) (VAM200) (EMG200) Mainframe MT2402 MT2400 (four channels Stim Rehab Bio (MT2200 electrotherapy +EMG module) +MTM200) MT2403 Mainframe (four channels MT2400 Stim Rehab Plus electrotherapy +battery module) (MT2200 +MTM200) UT2200 Mainframe UltraRehab (two channels ultrasound)...
- Page 10 No modification of this equipment is allowed! Do not try to open or remove the protective covers or disassembly the device for any reason.There is a danger of electric shock and serious injury.All service actions must be done by an authorized Nu-Tek service only;otherwise Nu-Tek bear no responsibility for further operation of the device.
- Page 11 Rehab-Series CAUTION: Keep yourself informed of the contraindications. Read the User’s Manual carefully and become familiar with all its safety requirements, operating procedures and maintenance instruction prior to use of the device.It is pro- hibited to use the device and its accessory in any manner that is not in accordance with the User’s Manual.
-
Page 12: Intended Use
Rehab-Series The device is designed to only be used by or under the supervision of persons using the medical device in the course of their work and in the framework of a professional healthcare activity, who understand the benefits and limitations of electrotherapy and ultrasound therapy. - Page 13 Rehab-Series Do not apply stimulation over open woundsor rashes, or over swollen, red, infected, or inflamed areas or skin eruptions (e.g., phlebitis, thrombophlebitis, varicose veins); Do not apply stimulation over, or in proximity to, cancerous lesions; Do not apply stimulation in the presence of electronic monitoring equipment (e.g., cardiac monitors, ECG alarms), which may notoperate properly when the electrical stimulation device is in use;...
- Page 14 Rehab-Series Use caution if stimulation is applied over the menstruating or pregnant uterus; Use caution if stimulation is applied over areas of skin that lack normal sensation. Isolated cases of skin rash may occur at the site of electrode placement following long-term applications.
- Page 15 Rehab-Series 4.1.2.2 Contra-indications Muscle Stimulation Do not use this device on patients who have a cardiac pacemaker, implanted defibril- lator, or other implanted metallic or electronic device, because this may cause electric shock, burns, electrical interference, or death. 4.1.2.3 Warnings Muscle Stimulation If you are in the care of a physician, consult with your physician before using this device;...
- Page 16 Rehab-Series Since the effects of stimulation of the brain are unknown, stimulation should not be applied across your head, and electrodes should not be placed on opposite sides of your head; The safety of electrical stimulation during pregnancy has not been established; You may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical conductive medium (gel);...
- Page 17 Rehab-Series These waveforms are often applied in combination with a surge program, which consists of a sequence of exercise and rest periods. Two options are available here: Reciprocal application, where stimulation alternates between agonists and antago- nists. This is accomplished through asynchronous stimulation over two current chan- nels with an appropriate delay between the two channels.
- Page 18 Rehab-Series Parameters: Carrier frequency: Carrier frequency is the base frequency of the alternating current. Beat Frequency: The frequency at which the amplitude is modulated. This is the effective therapeutic frequency. Vector-Auto: Vector-Auto is a form of amplitude modulation and is a percentage of the interferential amplitude (intensity) and will decrease from its maximum level over 6 seconds.
- Page 19 Rehab-Series Parameters: Phase Duration: expressed in µs, is the elapsed time from the beginning to the end of the initial pulse phase. Frequency: In a pulsed current the Frequency refers to the number of pulses that occur in a one second period of time and is denoted as in Hz or Pulses Per Second (pps). Frequency Modulation: expressed in Hz, defines a variable frequency range that is summed to the Pulse frequency i.e when the Pulse frequency is set to 80 Hz and the Frequency modu- lation is set to 40 Hz, the final frequency will vary from 80 –...
- Page 20 Rehab-Series The High Volt waveform is frequently used to increase local blood circulation and relax muscles in spasm. Frequency: In a pulsed current the Frequency refers to the number of pulses that occur in a one second period of time and is denoted as in Hz or Pulses Per Second (pps). Polarity: This refers to the polarity (+/-) of the red lead wire;...
- Page 21 Rehab-Series 4.1.3.8 Diadynamic Currents The Diadynamic waveforms are rectified alternating currents. The alternating current is modified (rectified) to allow the current to flow in one direction only. MF: (Monophasé Fixe) - Frequency of 50 Hz: phase duration of 10 ms followed by a pause of 10 ms.
- Page 22 Rehab-Series Polarity: This refers to the polarity (+/-) of the red lead wire; connect the lead wire to the active electrode. Cycle Time: refers to the time that the current is On and Off (in seconds). Example: for a Cycle Time of 10/50, the current will be flowing for 10 seconds and resting for 50 seconds. Ramp: The time that the current will take to increase to the set intensity level.
- Page 23 Rehab-Series Biphasic Pulsed Current TENS Phase duration Pulse frequency 200mA max. Asymmetrical Phase duration Pulse frequency 200mA max. Alternating Phase duration 200mA max. Pulse frequency Symmetrical Burst frequency 200mA max. Burst Asymmetrical Burst frequency 200mA max. Burst Symmetrical...
- Page 24 Rehab-Series Burst frequency 200mA max. Burst Alternating Phase duration: 2 ms 80mA max. Phase interval: 5 ms Träbert, 2 – 5 Current Phase duration 80mA max. Pulse frequency Rectangular Pulsed current Phase duration 80mA max. Pulse frequency Triangular Pulsed Current ...
- Page 25 Rehab-Series Burst frequency 200mA max. NMS Burst Galvanic Current Carrier frequency - 8 kHz fixed 80mA max. Duty cycle - 90 % fixed Galvanic Interrupted 80mA max. Galvanic Continuous Peak interval - 100 µs fixed 500V max. Pulse frequency High Voltage Frequency...
- Page 26 Rehab-Series Diadynamic Current 70mA max. 70mA max. 70mA max. 70mA max. CPid 70mA max. Modulation program Ramp Up Ramp Dowm Reset Time Surge program parameters...
- Page 27 Rehab-Series Frequence Modulation 1/Freq. Mod.+Frequency 1/Frequency TENS IPC-4P/Premod Beat High Beat Low Channel Mode CH1: CH2: Co-Cont Both(ON:10/OFF:10) CH1: CH2: Reciprocal(ON:10/OFF:10)
- Page 28 Rehab-Series 4.2 Intended Use Ultrasound therapy Ultrasound is a mechanical energy consisting of high-frequency vibrations applied by means of an ultrasound applicator. These vibrations pass through the tissue of the body and are gradually absorbed and transformed into heat. The resulting temperature increase triggers biological changes to occur in the tissue for the relief of pain, relaxation of muscle spasms and reduction of joint contractures.
- Page 29 Rehab-Series 4.2.3 Precautions and Warnings Ultrasound Precaution should be taken when using therapeutic ultrasound on patients with hem- orrhagic diatheses. Ultrasound treatment presents a potential safety hazard in patients whose pain response has been decreased because of disease, previous surgery, ionizing radiation therapy, chemotherapy, general or regional anaesthesia.
- Page 30 Rehab-Series Effective Radiation Area (ERA): expressed in cm², defines the cross-sectional area of the ultrasound beam (See technical specifications for details). The Effective Radiation Area is fixed and defined by the size of the ultrasound applicator. Ultrasound Power: is the ultrasound output expressed in Watt. The ultrasound output display can be toggled between Watt and Watt/cm².
- Page 31 Rehab-Series the capacity of a muscle to lengthen; This makes Myofeedback especially suitable as a measuring instrument for charting our locomotive operations. 4.4.1 Parameters Program time Max:99mins Threshold (µV) 0.6-2000µV, During work period the patient is prompted to contract above the Threshold.
-
Page 32: Package Contents
Rehab-Series Contraindications Because Biofeedback therapy does not “do” anything to the body, few contraindications exist. Biofeedback therapy is not recommended for persons with severe psychosis, depres- sion, or obsessional neurosis, nor for debilitated patients or those with psychopathic person- alities. However, because resulting functional improvements can require strenuous physical effort, individuals interested in Biofeedback may need to be aerobically fit. - Page 33 Rehab-Series 9051650010 Electrode Sponges(﴾80x120mm)﴿ 7100000081 Self-adhesive Electrodes(﴾50x50mm)﴿ 7100000152 Self-adhesive Electrodes(﴾50x100mm)﴿ 7200300010 Fixation strap(﴾75x1200mm)﴿ 7200300050 Fixation strap(﴾75x600mm)﴿ 7101000016 Stim Lead Wires 2003321 Holder for ultrasound applicator 9011032830 User manual 5.2 MT2200 Standard Accessories: Serial No. Name Quantity 1093294 MT2200 mainframe 1183323 Patient Interrupt Switch...
- Page 34 Rehab-Series 5.3 UT2200 Standard Accessories: Serial No. Name Quantity 1043305 UT2200 mainframe 7160132830 Mains Power cable 1811361 5cm2 ultrasound applicator 2240000006 Ultrasound Transmission Gel 1811373 1cm2 ultrasound applicator 2003321 Holder for ultrasound applicator 9011032830 User manual 5.4 MTM200 Standard Accessories: Serial No.
- Page 35 Rehab-Series 5.5 BTM200 Standard Accessories: Serial No. Name Quantity 1223315 BTM200 mainframe 5.6 VAM200 Standard Accessories: Serial No. Name Quantity 1223314 VAM200 mainframe 7900033220 Vacuum Electrode Cups Ø 60mm 7120033290 Vacuum Sponges Ø 60mm 7130033300 Vacuum Lead Hose (﴾Red)﴿...
- Page 36 Cart200. Ensure that there is sufficient air flow below the device (do not place the device on a table-cover). 6.1.2 Rehab series with a module (VAM200/ EMG200/ BTM200/ MTM200) Remove the functional module and any additional items ordered from the carton and inspect for damage that may have occurred during shipment.
- Page 37 Rehab-Series 6.2 Connection to mains supply Insert the mains cable into socket and connect it to a wall socket. CAUTION: Do not place the device in a location where the power cord could be tripped over or pulled out during treatment. Do not attempt to use the device if it is not properly grounded.
- Page 38 Rehab-Series 7 Application Information 7.1 Electrotheraphy CAUTION: Connection of accessories other than the ones specified by the manufacturer can adversely affect the safety of the patient and correct functioning of the equipment, and is therefore not permitted. To prevent infection, electrodes and sponge pads should not be used on broken skin. 7.1.1 Before treatment Check the patient for contra-indications and warnings as described in paragraph 4 Test the heat sensibility of the treatment area.
- Page 39 Rehab-Series 7.1.3Vacuum electrodes There is a choice of large and small electrodes. The areas of the electrodes correspond to those of the 4 x 6cm and 6 x 8cm flexible rubber electrodes. The vacuum electrodes are sufficiently flexible to ensure optimum contact with the skin, but rigid enough to prevent any changes in the contour of the part being treated, allowing full advantage to be taken of the massage effect of the pulsed vacuum.
- Page 40 Rehab-Series exceed a current density of 0.2 mA / cm². To find the maximum recommended current amplitude in mA for the Interferential, Premodu- lated and Russian Stimulation current waveforms, multiply the electrode surface in cm² by two. For all other current waveforms the stimulator output current can never exceed 50 mA r.m.s.
- Page 41 Rehab-Series 7.2.2 The contact medium To ensure efficient transfer of energy, a contact medium is required between the ultrasound applicator and the body. Air causes virtually total reflection of the ultrasound energy. The best medium for the transfer of ultrasound energy is a gel. The gel should be applied to the part of the body to be treated and then spread out with the ultrasound applicator.
- Page 42 Rehab-Series CAUTION: The ultrasound applicator is a precision instrument. Great care has been taken during the development and in production to obtain the best possible beam characteristics. Rough treatment (jarring or dropping) can adversely affect these characteristics, and must therefore be avoided. 7.2.5 After treatment Clean the skin of the patient and the ultrasound applicator with a towel or tissue.
- Page 43 Read carefully the probe instruction of use, originally attached to the probe pack- age. NOTE: Only NU-TEK, or appointed distributors /importers are approved to undertake servicing. Please contact us about our Vaginal /Rectal Probe. AC4001(24.5x97mm) NT1041(26.5x97mm) AC4000(29.5x97mm)
-
Page 44: Operating Instructions
Rehab-Series 8 Operating Instructions 8.1 Operator Controls [1] Mainframe of CT2200 [2] Power line switch 0...Device disconnected from mains supply 1...Device connected to mains supply [3] Connector for mains cable Type number/warning sticker Provides information on the apparatus, such as type and serial number, as well as connection data such as mains voltage and maximum current consumption. - Page 45 Rehab-Series [17] MTM200 module [18] Connection Electrode Cable Electrotherapy channel 4 [19] Connection Electrode Cable Electrotherapy channel 3 [20] BTM200 [21] VAM200 module [22] Connections Vacuum Cables Electrotherapy channel 1 [23] Connections Vacuum Cables Electrotherapy channel 2 [24] EMG200 module [25] Reference Electrode Cable channel [26] EMG Electrode Cable channel 1 [27] EMG Electrode Cable channel 2...
- Page 46 Rehab-Series CAUTION: Connection of accessories other than the ones specified by the manufacturer can adversely affect the safety of the patient and correct functioning of the equipment, and is therefore not permitted. For combined applications only use CARETALK type BF equipment.
- Page 47 Rehab-Series 8.3 Basic Operation 8.3.1 Device turn on Turn on the apparatus as described in paragraph 6.3 8.3.2 Display Organization The display is organized as a spreadsheet of 3 sheets, one for each channel. The channels refer to the patient connector groups accessible at the front of the unit. A sheet can be selected by touching its tab.
- Page 48 Rehab-Series A selected sheet gives an overview of the parameters belonging to that channel. A param- eter can be selected by touching it, which causes its sheet to be lit and the strip lamp in the bottom of the screen bar is to be illuminated. The parameter can now be adjusted with the To adjust the output amplitude of a channel, touch the tab of the selected channel again.
- Page 49 Rehab-Series Icon name Meaning Start/Continue treatment. The output current increases to the Start/Continue previous value and the treatment timer resumes counting down. Accept the selected option. Accept Stop Stop the treatment,reset the treatment time and intensity. 8.3.3.2 Channel Tab Information Channel Tab Illustration Of Electrotherapy ...
- Page 50 Rehab-Series Channel Tab Illustration Of Combination Output Indicator Standard electrodes I Output Indicator Ultrasound Applicator J Remaining treatment time. When a sequential protocol has been loaded, the value K indicates the total remaining treatment time of the sequential protocol. Output value L ...
- Page 51 Rehab-Series Electrotherapy “Clinical Protocols” The electrotherapy menu gives access to functions Select Clinical Protocols by touching the button. The next screen appears. Use the central controller to scroll through the list and select the clinical protocol by touching the button. The channel selection screen appears.
- Page 52 Rehab-Series Channel Selection Here you can select the channels for electrotherapy. When channel 1 is selected, channel 2 is still available for another therapy. When Channel 1+2 is selected both channels have the same parameters. Only the intensity can be set differently.
- Page 53 Rehab-Series Start the therapy by adjusting the intensity with the button in the navigation bar. button in the navigation bar. button in the navigation bar. Electrotherapy “Manual Operation” The electrotherapy menu gives access to functions Select Manual Operation by touching the button. The next screen appears.
- Page 54 Rehab-Series Channel Selection Here you can select the channels for electrotherapy. When channel 1 is selected, channel 2 is still available for another therapy. When Channel 1+2 is selected both channels have the same parameters. Only the intensity can be set differently.
- Page 55 Rehab-Series Start the therapy by adjusting the intensity with the button in the navigation bar. button in the navigation bar. button in the navigation bar. 8.2.4.2 Ultrasound Therapy The Home menu gives access to all functions of the unit.
- Page 56 Rehab-Series Use the central controller to scroll through the ist and select the clinical protocol by touching the button. For therapy information touch the info button left of the protocol and the therapy info will appear. ...
- Page 57 Rehab-Series 8.2.4.3 Combination Therapy This therapy combines ultrasound and electrotherapy. The Home menu gives access to all functions of the unit. Select in the Home menu combination therapy by touching the button “Combination”. The next screen appears. ...
- Page 58 Rehab-Series 8.2.4.3 Combination Therapy Adjust the current parameters and treatment time ultrasound parameters. the previous screen. adjust the current intensity. (electrode and treatment head need to be in contact with the patient). ...
- Page 59 Rehab-Series 8.2.4.4 Vacuum The Home menu gives access to all functions of the unit. Select in the Home menu vaccum therapy by touching the button “Vacuum” if a unit is equipped with a Vacotron it’s possible to select either. The next screen appears.
- Page 60 Rehab-Series 8.2.4.5 MTM200 4 channel electrical stimulation If a unit is equipped with MTM200,electrical stimulation increased to four channel. Here you can select the channels for electrotherapy. When channel 1 is selected, channel 2,3 and 4 are still available for another therapy. When Channel 1+2 or 3+4 is selected both channels have the same parameters.
- Page 61 Rehab-Series The Combination menu gives access to functions Select ETS by touching the button. The next screen appears. The EMG menu gives access to functions. Select Manual Operation by touching the button The next screen appears. ...
- Page 62 Rehab-Series Channel Selection Here you can select the channels for electrotherapy. When channel 1 is selected, channel 2 is still available for another therapy. When Channel 1+2 is selected both channels have the same parameters. Only the intensity can be set differently. ...
- Page 63 Rehab-Series 8.2.4.7 Storing Favorites When a treatment screen is completely set as required, its settings can be stored in a favorite for later use: a Store button is available on the navigation bar. To store your settings,touch the Store button ...
- Page 64 Rehab-Series In this screen you can personalize the unit. Several settings can be changed or adjusted. Touch the language button again or touch another button to confirm. of the backlight of the screen. Settings menu. to return to the Home menu. 8.2.5 Shutting device down Turn off the device as described in paragraph 6.4.
- Page 65 Rehab-Series 8.2.6.2 Adjusting current amplitude To adjust the output current, touch the tab of the selected channel. Its colour will change to The current amplitude can only be adjusted when the clock has been set. With 4 polar interferential current waveforms the current amplitude operates on both chan- nels simultaneously.
-
Page 66: Maintenance And Troubleshooting
Rehab-Series 9. Maintenance and Troubleshooting 9.1 Cleaning 9.1.1 Cleaning of the device Switch off the device and disconnect it from the power supply.The apparatus can be cleaned with a damp cloth. Use lukewarm water and a non-abrasive liquid household cleaner (no abrasive, no alcohol content solution). If a more sterile cleaning is needed, use a cloth moistened with an antimicrobial cleaner. - Page 67 Rehab-Series CAUTION: The electrodes are intended for single patient use only . If irritation occurs, discontinue use and consult your clinician. Always use the electrodes with CE mark, or are legally marketed in the US under 510(K) procedure. 9.1.4 Cleaning the lead wires and cables Periodically wipe the lead wires clean with a cloth dampened in a mild soap solution, and then gently wipe them dry .
- Page 68 Rehab-Series 9.1.8 Cleaning Vaginal/Rectal probes Carefully clean the probe after use.Wash the probe gently in mild soapy water,rinse and make sure the probe is completely dry before returning to storage in the plastic bag. 9.1.9 Cleaning the water reservoir and hoses Detach the vacuum cups from the vacuum cables.
- Page 69 Rehab-Series 9.2.2 Warning tone Content System error code reason processing Tooltip Voice Electrical stimulation Bad contact quality on Warning System Stop channel 1 is without channel 1.Check pads tone Electrical load and lead wires. stimulation output Electrical stimulation Bad contact quality on Warning System Stop channel 2 is without...
- Page 70 Rehab-Series System detect Ultrasound Attempting to perform System Stop Warning handle is not connect Ultrasound treatment Ultrasound tone when the Ultrasound with no Applicator treatment output treatment is selected. connected to the system Ultrasound applicar Ultrasound Applicator Warning System Stop overheat is too hot.
- Page 71 Rehab-Series Vacuum adsorption There probably is Warning The operation module e leak a leak in the vacuum tone is invalid system. Device inside System is too hot. Warning System stop Overtemperature tone output Battery overtemperature Battery is too hot. Warning System stop tone output...
- Page 72 The manufacturer will not be held responsible for the results of maintenance or repairs by unauthorized persons. All other technical maintenance is restricted to authorized Nu-Tek maintenance personnel. Authorized service personnel can make use of 1498770 Service manual REHAB SERIES 9.4 Troubleshooting...
- Page 73 Unknown Contact clinician 9.5 End of life The REHAB SERIES contains materials that can be recycled and/or are noxious to the environment. Specialized companies can dismantle the unit and sort out these materials. When you dispose of the unit, find out about local regulations concerning...
- Page 74 Rehab-Series 10. Specifications 10.1 Ultrasound parameters Frequency.................1 MHZ,±10%;3 MHZ,±10% Duty Cycles.............10%-100%,Continues,stepping 10% Pulse duration............1 – 9 ms ± 10 % (set by duty cycle) PulseFrequency..................16,48,100Hz Output Power ²................0.5W-10.0W ²................0.5W-15.0W ²................0.1W-2.0W ²................0.1W-2.0W Output accuracy........± 20% (for any level above 10% of maximum) Peak out Amplitude Duty Treatment timer................0 - 30 min ±...
- Page 75 Rehab-Series 10.2 Stimulator output parameters 10.2.1 IFC-4P:IFC(Interferential) Traditional (4 Pole) Interferential Current is a medium frequency waveform. Current is distributed through of two channels (four electrodes). The currents cross each other in the body at the area requiring treatment. The two currents interfere with each other at this crossing point, resulting in a modulation of the intensity (the current intensity increases and decreases at a regular frequency).
- Page 76 Rehab-Series 10.2.3 Biphasic (TENS) The Asymmetrical Biphasic and the Symmetrical Biphasic waveform are often used in TENS (Transcutaneous Electrical Nerve Stimulation) applications. The TENS has a short pulse duration. It is capable of strong stimulation of the nerve fibers in the skin as well as of muscle tissue.
- Page 77 Rehab-Series Channel mode....Single channel Mode,Reciprocal Mode,Co-Contraction Mode Treatment Time..................1-60 minutes Cycle Time........continues,10/10,10/20,10/30,10/50,custom:0-60 Ramp.................0/0,1/1, 2/2,5/5,8/8,custom:0-9 10.2.3.4 TENS Symmetrical Burst Frequency..................1-250Hz, stepping 1Hz Burst ..................1-9bps, stepping 1bps P.Dur..................20us-1000us,stepping 5us Intensity........CC:0-200mA,stepping 0.5mA;CV:0-100V,stepping 0.5V Channel mode....Single channel Mode,Reciprocal Mode,Co-Contraction Mode Treatment Time..................1-60 minutes Cycle Time........continues,10/10,10/20,10/30,10/50,custom:0-60 Ramp.................0/0,1/1, 2/2,5/5,8/8,custom:0-9 10.2.3.4 TENS Alternating Rec.
- Page 78 Rehab-Series 10.2.4 Russian Russian Current is a rectangle waveform, delivered in bursts or series of pulses. This method was claimed by its author (Kots) to produce maximal muscle strengthening effects without significant discomfort to the patient. Carrier Freq....................2-10KHz Burst Frequency.................20-100Hz,stepping 5Hz Duty cycle.................10%-50%,stepping 10% Intensity........CC:0-200mA,stepping 0.5mA;CV:0-200V,stepping 0.5V Channel mode....Single channel Mode,Reciprocal Mode,Co-Contraction Mode...
- Page 79 Rehab-Series Phase Duration..................Positive,negative Phase Duration.......................2ms Interval........................5ms Intensity.........CC:0-70mA,stepping 0.5mA;CV:0-70V,stepping 0.5V Treatment Time..................1-60 minutes 10.2.6.2 Rectanglar Frequency..............0.2Hz-200Hz.stepping 0.1Hz/1Hz Polarity....................Positive,negative P.Dur..............20-10000us,steping 100us/1000us Intensity.........CC:0-80mA,stepping 0.5mA;CV:0-80V,stepping 0.5V Channel mode....Single channel Mode,Reciprocal Mode,Co-Contraction Mode Treatment Time..................1-60 minutes Cycle Time........continues,10/10,10/20,10/30,10/50,custom:0-60 Ramp.................0/0,1/1, 2/2,5/5,8/8,custom:0-9 10.2.6.3 Triangular Frequency..............0.2Hz-200Hz.stepping 0.1Hz/1Hz Polarity....................Positive,negative P.Dur..............20-10000us,steping 100us/1000us Intensity.........CC:0-80mA,stepping 0.5mA;CV:0-80V,stepping 0.5V Channel mode....Single channel Mode,Reciprocal Mode,Co-Contraction Mode...
- Page 80 Rehab-Series Cycle Time........continues,10/10,10/20,10/30,10/50,custom:0-60 Ramp.................0/0,1/1, 2/2,5/5,8/8,custom:0-9 10.2.8 High Voltage The High Voltage Pulsed Current (HVPC) has a very brief pulse duration characterized by two distinct peaks delivered at high voltage. The waveform is monophasic (current flows in one direction only). The high voltage causes a decreased skin resistance making the current comfortable and easy to tolerate.
- Page 81 Rehab-Series 10.2.10.2 Interrupted Frequency.....................8KHz fixed Polarity....................Positive,negative Duty cycle......................90% fixed Intensity..........CC:0-80mA,stepping 5uA;CV:0-80V,stepping 5V 10.3 Parameter Limit For security, the device has some some limited for electrotherapy.The maximum intensity has a relationship with the frequency and pulse duration following below table: 10.4 Technical Data Power supply..............100V-240V, 47Hz-63Hz, 1.35A Power output....................15V , 4A Max Dimensions................256x180x124mm(LxWxH)
- Page 82 Rehab-Series 10.6 Safety and Performance standards IEC 60601-1 General requirements for the safety of electrical medical systems, including Annex 1, national differences for Australia, Canada and the United States. Safety class according to IEC 60601-1 class I type BF IEC 60601-2-5 Particular requirements for the safety of ultrasonic therapy equipment.
- Page 83 Guidelines and manufacturer's declaration – electromagnetic interference The REHAB SERIES device is intended for operation in an electromagnetic environment as indicated below. The customer or user of the REHAB SERIES unit should ensure that it is operated in such an environment.
- Page 84 Guidance and manufacturer’s declaration – Electromagnetic immunity The REHAB SERIES device is intended for use in the electromagnetic environment specified below. The customer or the user of the REHAB SERIES device should assure that it is used in such an environment.
- Page 85 Guidelines and manufacturer's declaration – electromagnetic interference immunity The REHAB SERIES device is intended for operation in the electromagnetic environment specified below. The customer or user of the REHAB SERIES should ensure that it is used in such an environment.
- Page 86 If the measured field strength in the location where the REHAB SERIES device is to be used exceeds the above compliance levels, the REHAB SERIES device should be monitored in order to ensure that it is functioning as intended.
- Page 87 Rehab-Series Separation distance according to frequency of transmitter m Rated output of transmitter 150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz 0.01 0.12 0.035 0.07 0.38 0.11 0.22 0.35 0.70 For transmitters rated at a maximum output which is not listed above, the recommended separation distance d in meters (m) can be determined using the equation applicable to the respective column, whereby P is the maximum rated output of the transmitter in Watts (W) according to the transmitter manufacturer.
-
Page 88: Ordering Information
Rehab-Series 11. Ordering information Model numbers: 11.1 CT2200 Standard Accessories: Serial No. Name Quantity 1053283 CT2200 mainframe 1183323 Patient Interrupt Switch 7160132830 Mains Power cable 1811361 ultrasound applicator 2240000006 Ultrasound Transmission Gel 7100100001 Rubber electrodes(﴾60x90mm)﴿ 7100100000 Rubber electrodes(﴾70x110mm)﴿ 9051650011 Electrode Sponges(﴾70x100mm)﴿ 9051650010 Electrode Sponges(﴾80x120mm)﴿... - Page 89 Rehab-Series 11.2 MT2200 Standard Accessories: Serial No. Name Quantity 1093294 MT2200 mainframe 1183323 Patient Interrupt Switch 7160132830 Mains Power cable 7100100001 Rubber electrodes(﴾60x90mm)﴿ 7100100000 Rubber electrodes(﴾70x110mm)﴿ 9051650011 Electrode Sponges(﴾70x100mm)﴿ 9051650010 Electrode Sponges(﴾80x120mm)﴿ 7100000081 Self-adhesive Electrodes(﴾50x50mm)﴿ 7100000152 Self-adhesive Electrodes(﴾50x100mm)﴿ 7200300010 Fixation strap(﴾75x1200mm)﴿ 7200300050 Fixation strap(﴾75x600mm)﴿...
- Page 90 Rehab-Series 11.4 MTM200 Standard Accessories Serial No. Name Quantity 1223317 MTM200 mainframe 1183323 Patient Interrupt Switch 7100100001 Rubber electrodes(﴾60x90mm)﴿ 7100100000 Rubber electrodes(﴾70x110mm)﴿ 9051650011 Electrode Sponges(﴾70x100mm)﴿ 9051650010 Electrode Sponges(﴾80x120mm)﴿ 7100000081 Self-adhesive Electrodes(﴾50x50mm)﴿ 7100000152 Self-adhesive Electrodes(﴾50x100mm)﴿ 7200300010 Fixation strap(﴾75x1200mm)﴿ 7200300050 Fixation strap(﴾75x600mm)﴿ 7101000016 Stim Lead Wires ...
- Page 91 Rehab-Series 11.7 EMG200 Standard Accessories Serial No. Name Quantity 1223316 EMG200 mainframe 7100000081 Self-adhesive Electrodes(﴾50x50mm)﴿ 10260041 Intravaginal Probe(﴾26.5mm)﴿ 7101000016 Stim Lead Wires 7101000017 EMG Leadwire Optional Accessories: Serial No. Name Quantity 11811670 Intravaginal Probe(﴾29.5mm)﴿ 10660042 Anal Probe 11.8 Cart: Serial No.


Need help?
Do you have a question about the Rehab Series and is the answer not in the manual?
Questions and answers
Hi. Do you have Russian instruction or Ukrainian version on PDF format or something like it?
Need the reset password