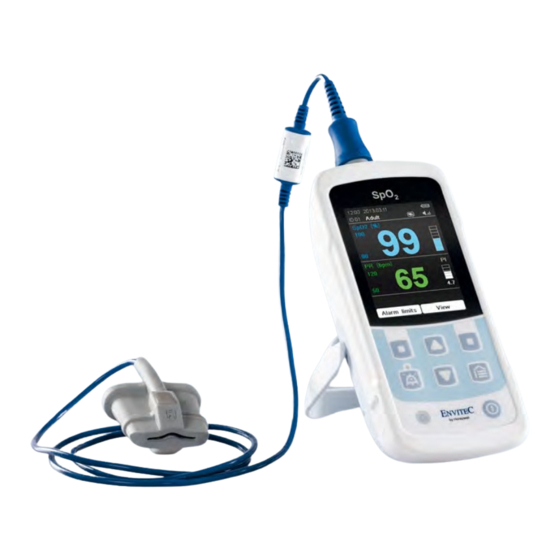
Summary of Contents for Envitec MySign s
- Page 1 Pulse oximeter Technical Documentation Subject to technical changes! 066-07-1002197_GA_MySignS_FDA-0 © 06.2014 EnviteC-Wismar GmbH...
-
Page 2: Table Of Contents
6.8.6 Pulse sound ..........................35 Info ..................................36 6.9.1 System information ........................36 6.9.2 Device personalization ......................... 36 EnviteC SpO2 sensors and accessories ....................... 37 Selection and application of a sensor ......................37 Overview of EnviteC sensors and cables ......................38 066-07-1002197_GA_MySignS_FDA / 06.14... - Page 3 Pulse oximeter MySign ® Maintenance / cleaning ............................39 Replacing the battery ............................40 Replacing the sensor ............................40 PC software ................................41 Alarm messages ..............................42 Info ..................................... 43 Error Descriptions and Troubleshooting ......................... 43 Technical specifications ............................44 13.1 Clinical Accuracy ..............................
- Page 4 Reprinting, translation and reproduction in any form – even excerpts – require the written approval of the manufacturer. This manual is subject to revision by EnviteC-Wismar GmbH. The latest edition of this technical documentation can be downloaded from our web page www.envitec.com.
-
Page 5: General Information
70 °C. Maintenance The pulse oximeter must only be maintained and serviced by the hospital/dealer's technical service personal or by EnviteC-Wismar GmbH staff. Disposal In accordance with Directive 2002/96/EC (WEEE), the manufacturer will accept the return of the electrical and electronic device for proper disposal after dismantling! MR Unsafe MR Unsafe –... -
Page 6: Safety Information
Incorrect use or positioning of the sensors can result in measurement errors and can lead to constriction of body parts by the sensor cable, shearing off of skin portions by the sensor, etc. • Only the sensors and accessory parts approved by EnviteC-Wismar GmbH may be used with the MySign ®... - Page 7 EnviteC only considers itself responsible with regard to the safety, reliability and function of the units if the assembly, extensions, reconfigurations, changes and repairs are performed by EnviteC or by a party expressly authorized for this by EnviteC and the unit is used in accordance with the operating manual and the technical documentation.
- Page 8 Pulse oximeter MySign ® Essential Performance Essential Performance is performance, the absence or degradation of which, would result in an unacceptable risk, and includes the following functionalities: • Accuracy of blood oxygen saturation (SpO2) measurement • Accuracy of pulse rate measurement •...
-
Page 9: Designated Use And Functional Description
Pulse oximeter MySign ® Designated use and functional description Indications for use (designated purpose) MySign ® S is a handheld pulse oximeter with accessory sensors indicated for continuous non-invasive monitoring of the functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate for adult and pediatric (excluding neonatal and infant) patients in hospitals, hospital-type facilities, and mobile units. -
Page 10: General Functional Principles And Conditions
Pulse oximeter MySign ® General functional principles and conditions The technology of non-invasive pulse oximetry is based on two principles. On one hand, the color of the blood influenced by oxygen saturation is determined at the two wavelength ranges of red and infra-red (spectrophotometry). - Page 11 Pulse oximeter MySign ® The artifact leveling (AL) is used to suppress movement artifacts for the SpO2 and pulse rate parameters. In addition, the pulse rate is checked for plausibility with a deviation suppression (DS) function. ® The MySign S is calibrated to pulse-oximetric hemoglobin oxygen saturation with dyshemoglobin- free blood based on reference measurements via fractional saturation measurement (CO-oximeter).
-
Page 12: Commissioning
If the shipping box is damaged, notify your shipper. Unpack the MySign ® S and its accessories. If a part is missing or damaged, contact the customer service of EnviteC or your local EnviteC dealer. Parts list ® 1 x pulse oximeter MySign... -
Page 13: Charging The Battery
The battery can only be charged at an external power supply using a suitable wall power supply with a USB port (EnviteC Part No.: 1001829). When using the EnviteC external power supply, charging the battery takes around 4 hours and is completed as soon as the battery symbol is shown as full. -
Page 14: Set Up / Mounting
Pulse oximeter MySign ® Set up / mounting The MySign ® S must be set up / mounted as per the requirements at the place of use, on an even surface or using the universal holder (optional, Part No. 1001801) for all profiles. Fold out the support provided on the MySign ®... -
Page 15: Operating Elements And Symbols
Pulse oximeter MySign ® Operating elements and symbols The system is operated using the membrane keys on the front. All of the system's status and error messages are shown in plain text on the illuminated screen. Front Back Description Description Housing ON/OFF key Display... -
Page 16: Keys And Leds
Pulse oximeter MySign ® Keys and LEDs Function keys 1 + 2 (depending on menu) Example: Function 1 Function 2 Select key UP For selecting menu items and changing parameters (Pulse sound louder) Select key DOWN For selecting menu items and changing parameters (Pulse sound quieter or off) Main menu or home key... -
Page 17: Display
Pulse oximeter MySign ® Display Description Date and time indicator Patient type: Shows the preset patient type (adult or child). Also see section “Patient type”. ID (identification): Shows the current measurement series number. Also see section “Measurement series” Symbols: See additional table for explanation (5.4) Battery status indicator: See section “Alarm messages”... -
Page 18: Symbols On The Label
Pulse oximeter MySign ® Symbols on the label Follow the instructions given in the IP54 Protected against spray water and dust operating manual! Manufacturer + date of Unit corresponds to type BF – Not protected manufacture from the effects of defibrillators Product number Follow the disposal instructions! Serial number... -
Page 19: Symbols Shown On The Display
Pulse oximeter MySign ® Symbols shown on the display Key lock ON Mains operation/charge Key lock OFF Connecting the system to a computer View Flag for marking within a dataset Data memory Error (yellow = minor / red = severe) Alarm condition Alarm settings (Symbol to display an alarm condition) -
Page 20: Operation
Pulse oximeter MySign ® Operation All functions of the unit and the necessary initial configuration are described in this section. Switching the unit on / off Once the unit has been switched on, it will automatically test all of its internal functions and components. While performing these tests, the symbol “Follow the operating manual”... - Page 21 Pulse oximeter MySign ® If this prompt regarding copying the personalized measurement data is not confirmed within 2 minutes, they are not copied. In that case, the system will generate a new measurement series record. Switching off the unit The unit will show a countdown for the shutdown process, starting with the number 3. If the ON/OFF key is released at any time during the countdown, the countdown is stopped and the unit stays on.
-
Page 22: Main Menu
Pulse oximeter MySign ® Main menu Overview 066-07-1002197_GA_MySignS_FDA / 06.14... -
Page 23: Key Lock
Pulse oximeter MySign ® Key lock For this, the keys are locked to prevent changes from being made to the device settings. Select “Key lock” from the main menu. Deactivate the key lock by pressing “Unlock ?”. Confirm the subsequent query by pressing “OK”. The keys are now unlocked and can be used to operate the unit. -
Page 24: Patient Type
Pulse oximeter MySign ® Patient type Various initial settings can be configured for specific groups of persons in the “Patient type” menu. Adult SpO2 alarm limits: Upper Lower 100 % 90 % Pulse alarm limits: Upper Lower 120 bpm 50 bpm Child SpO2 alarm limits: Upper... -
Page 25: Measured Value
Pulse oximeter MySign ® 6.5.1 Measured value In this display mode, the currently measured SpO2 and pulse values as well as the pulsation index and signal quality are displayed. To view an overview of the measurements that have already been performed, go to “Measurement series”... -
Page 26: Ptm
Pulse oximeter MySign ® 6.5.2 PTM In this mode, the unit will show both the “Measured value” and “Plethysmogram”. Select “PTM” from the “View” menu. Horizontal position PTM view When holding the unit horizontally, the PTM view will also be displayed horizontally. 066-07-1002197_GA_MySignS_FDA / 06.14... -
Page 27: Trend
Pulse oximeter MySign ® 6.5.3 Trend In this mode, the system will show the current measurement and a time line showing the trend of the measurements taken over the past three hours. This gives a comprehensive overview of any changes in the measurements. -
Page 28: Data Memory
Pulse oximeter MySign ® Data memory The data memory contains the data from previous measurements. These recordings are listed by ID or chronologically by start and end date. Select “Data memory” from the main menu. Once the “Data memory” menu has opened, you will be able to select from a number of different functions. -
Page 29: Measurement Series
Pulse oximeter MySign ® 6.6.3 Measurement series A measurement series comprises all of the measurements (SpO2 and pulse) that are taken during one measuring cycle. A new measuring cycle is started every time the unit is switched off and on, and each new series of measurements is assigned a separate ID. -
Page 30: Alarm Settings
Pulse oximeter MySign ® Alarm settings The alarm settings contain all configuration options for alarms. Select “Alarm settings” from the main menu. Once the “Alarm settings” menu has opened, you will be able to select from a number of different options. 066-07-1002197_GA_MySignS_FDA / 06.14... -
Page 31: Alarm Limits
Pulse oximeter MySign ® 6.7.1 Alarm limits The alarm limits are the upper and lower limits for SpO2 and pulse such that an integrated signal device will indicate an alarm if the values are outside of this range. Select “Alarm limits” from the “Alarm settings” menu using the select key. -
Page 32: Audio Alarm
Pulse oximeter MySign ® 6.7.3 Audio alarm This is where the audio alarm for all messages can be switched on and off. Select “Audio alarm” from the “Alarm settings” menu. This is switched on or off with the right function key. Other options: 1. -
Page 33: Application Indicator
Pulse oximeter MySign ® 6.7.5 Application indicator Indicates how long the SpO2 sensor has been used at the same application point (e.g. index finger). The application indicator is given in hours from 0 - 8. The display starts as soon as the sensor is placed or re- applied. -
Page 34: General Settings
Pulse oximeter MySign ® General settings The unit's main settings can be configured in the “General settings” menu. The settings can also be configured using the supplied software. Select “General settings” from the main menu. Once the “General settings” menu has opened, you will be able to select from a number of different options. -
Page 35: Reset Device (Factory Setting)
Pulse oximeter MySign ® 6.8.4 Reset device (factory setting) Here, the unit can be reset to the factory settings or user settings. The user settings are defined using the PC software. Factory settings Pulse sound Alarm sound Alarm sound volume Reminder signal Application indicator Key sound... -
Page 36: Info
Pulse oximeter MySign ® Info This menu can be used to call up information about the unit and the sensor. Select “Info” in the main menu. Once the “Info” menu has opened, you will be able to select from a number of different options. 6.9.1 System information The system information menu contains the most important data about the system. -
Page 37: Envitec Spo2 Sensors And Accessories
Never use damaged sensors. Never use a sensor with unprotected optical components. Only use sensors approved by EnviteC for SpO2 measurements. When selecting the sensor, consider the weight and activity level of the patient. Also evaluate whether there is sufficient blood circulation at the application site. -
Page 38: Overview Of Envitec Sensors And Cables
A list of the part numbers can be found in the section “Order information”. A current list of the approved sensors can be found at www.envitec.com. 066-07-1002197_GA_MySignS_FDA / 06.14... -
Page 39: Maintenance / Cleaning
The unit also continuously monitors all of its functions while operating. Always check the unit, the sensor, the charging equipment and all cables for external damage before use. The unit may only be serviced by EnviteC or service personnel that have been trained by EnviteC. Repairs... -
Page 40: Replacing The Battery
5 minutes while the battery is being replaced. After this time, all settings must be reconfigured. Disposing of the unit, SpO2 sensor, battery Do not dispose of the unit, SpO2 sensor or battery as household waste. Please return all of these to EnviteC clearly labelled “Entsorgung (Please dispose of)”. EnviteC-Wismar GmbH... -
Page 41: Pc Software
Pulse oximeter MySign ® PC software ® The integrated USB port can be used for exchanging data between a computer and MySign monitor. The steps for connecting the relevant cables for exchanging data and for charging the battery from the computer port are identical (see section “Commissioning”). -
Page 42: Alarm Messages
Pulse oximeter MySign ® Alarm messages Audio Visual signals Description Priorities signals Charge level of the battery too low Glowing (remaining time about 2 hours) yellow The battery must be charged as soon as possible. Critical charge level of the battery Flashing (remaining time about 15 min.) yellow,... -
Page 43: Info
Pulse oximeter MySign ® Info "Information field" display Cause Remedy Pulsation index (PI) below 1 Improve blood flow or select another location to apply the sensor. < 1 Flashing Poor blood flow at the application point. As soon as a pulse signal has Indication that the pulse signal has Pulse search been detected, this message will... -
Page 44: Technical Specifications
Pulse oximeter MySign ® Technical specifications All of the specifications apply to the following standard conditions: Ambient temperature of 1013 hPa, 25°C dry ambient air. Display area : Saturation (SpO2) 1 to 100% Pulse rate (PR) 0 to 300 BPM Pulsation index (PI) below 0.1 to 20 Precision* : Saturation +/- 2% (70 to 100%, without disrupting movement) - Page 45 Pulse oximeter MySign ® Alarm limits : Can be adjusted to between SpO2: Upper limit: 51 % - 100% Lower limit: 50 % - 99% Pulse: Upper limit: 31 - 250 BPM Lower limit: 30 – 249 BPM Alarm sound : 55 –...
-
Page 46: Clinical Accuracy
• DW-2211-6 Disposable Adhesive Tape Sensor • DS100A Nellcor Finger Clip Sensor (NOTE: third party sensor not manufactured by EnviteC) In total the sensor types above are representative of the construction of the MySign ® S full sensor product offerings for FingerClip, EarClip, SoftTip, and disposable sensors. - Page 47 Pulse oximeter MySign ® F-3212-9 Reusable FingerClip SpO2Sensor ES-3212-9 Reusable EarClip SpO2 Sensor 066-07-1002197_GA_MySignS_FDA / 06.14...
- Page 48 Pulse oximeter MySign ® R-3212-9 SoftTip Reusable Rubber Finger Sensor DW-2211-6 Disposable Adhesive Tape Sensor 066-07-1002197_GA_MySignS_FDA / 06.14...
- Page 49 DS100A Nellcor Finger Clip Sensor The third party Nellcor DS-100A has been validated for use on the MySign S. The DS-100A is not manufactured by EnviteC. Refer to the manufacturer’s DS-100A instructions for use for more information on this sensor.
-
Page 50: Warranty
® Warranty As of the purchase date, EnviteC offers a two year warranty for faults arising from material or manufacturing defects. Excepted from this are the battery and the SpO2 sensor (see the General Terms of Business). Faults that are covered by warranty will be corrected within the framework of our warranty conditions. EnviteC... -
Page 51: Order Information
Pulse oximeter MySign ® Order information Description Part number MySign ® FDA without Sensor 1002197 Accessories Data cable (Mini-USB type B) 1001815 ® MySign battery 1001734 MySign ® power supply (Mini-USB 5 V / 1.5 A) 1001829 (optional) MySign ® holder 1001801 (optional) ®... - Page 52 Pulse oximeter MySign ® SpO2 sensors with adapter cable Part number Extension and adapter cables 1002208 X-4211-3 MySign ® Extension and adapter cables 1002209 ® X-4211-1 MySign SoftTip ® Large R-3212-9 MySign ® 1002210 SoftTip ® Medium RM-3212-9 MySign ® 1002211 ®...
-
Page 53: Emc Declaration
Table 1: Guidance and manufacturer`s declaration - electromagnetic emissions Guidance and manufacturer`s declaration electromagnetic emissions The MYSIGN S is intended for use in the electromagnetic environment specified below. The customer or the user of the MYSIGN S should assure that it is used in such an environment. - Page 54 Table 2 and 4: Guidance and manufacturer`s declaration - electromagnetic immunity Guidance and manufacturer`s declaration electromagnetic immunity The MYSIGN S is intended for use in the electromagnetic environment specified below. The customer or the user of the MYSIGN S should assure that it is used in such an environment.
- Page 55 To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic side survey should be considered. If the measured field strength outside the location in which the MYSIGN S is used exceeds the compliance level, the MYSIGN S should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as relocating or using another location of the MYSIGN S.
- Page 56 EnviteC-Wismar GmbH a Honeywell Company Alter Holzhafen 18 Phone: 49 - (0) 3841 360-200 23966 Wismar, Germany Fax: 49 - (0) 3841 360-222 Internet: www.envitec.com...



Need help?
Do you have a question about the MySign s and is the answer not in the manual?
Questions and answers