Summary of Contents for Circassia NIOX VERO
- Page 1 NIOX VERO ® Airway Inflammation Monitor User Manual 510(k) 133898 000190-09 (EPM-000165) NIOX VERO ® User Manual US April 2017...
- Page 2 CAUTION!: Do not use NIOX VERO ® in the proximity of areas where there is a risk for condensation of water inside NIOX VERO. volatile substances such as organic fluids or disinfectants are being Normally a maximum of 10 exhalations/hour can be performed with used.
-
Page 3: Table Of Contents
® 6.2 Installation of NIOX Panel ............ 23 ® 1.1 Before using NIOX VERO Airway Inflammation Monitor ..3 6.3 Connect to a PC via USB............24 1.2 About this manual..............3 6.4 Connect to a PC via Bluetooth ..........24 1.3 Compliance ................ - Page 4 11.16 Instructions for transport and storage......... 45 ® 12 NIOX VERO parts and accessories ........46 ® 12.1 Parts included in NIOX VERO package (Article No. 12-1200) ..............46 12.2 Accessories ................46 13 Medical Device Reporting (MDR) .........47 14 Guidance and manufacturer's declaration - Electromagnetic...
-
Page 5: Important Information
Chapter 1 Important information Important information ® The User Manual provides instructions on how to operate NIOX VERO It contains numbered step-by-step instructions with screens and illustrations. Choices within steps are displayed with bullet points. Before using NIOX VERO ®... -
Page 6: Indications For Use
American Thoracic Society. components. Measurement of FeNO by NIOX VERO is a quantitative, non-invasive, sim- • Keep the instrument and Sensor out of water. Ensure that no liquid is ple and safe method to measure the decrease in FeNO concentration in spilled or dropped on the instrument or Sensor. -
Page 7: Product Description
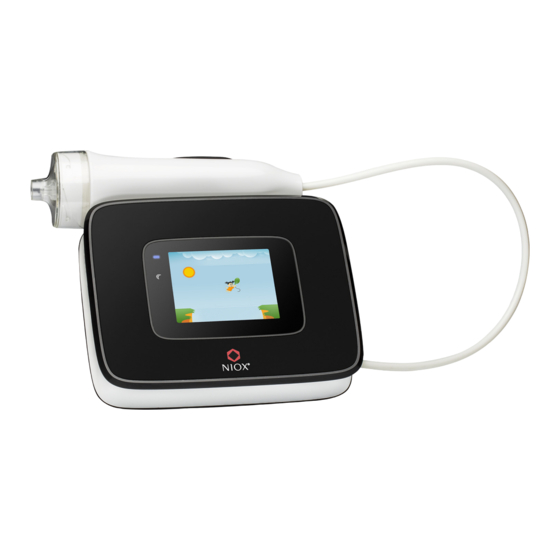
USB memory stick, (F) USB cable, (G) Power adapter and power cord, (H) Patient filter (supplied separately) Note: Only accessories and parts supplied by Circassia may be used. (K) Battery LED - lit when battery is charging, (L) Standby LED - blinking in Standby/Sleep mode, (M) Touch panel Display ®... -
Page 8: Installation And Set Up
There is a taper on the side of the lid for better grip when opening. Open the Sensor can. (N) Breathing handle holder, (O) Breathing handle port ® 000190-09 NIOX VERO User Manual US... - Page 9 Open the Sensor package. Note: Only use the correct rechargeable battery supplied by Circassia. Type No BJ-G510039AA, Article No 12-1150 WARNING! Do not touch or clean the white Sensor membrane. CAUTION! The Sensor should only be stored in its original unopened package or installed in a NIOX VERO ®...
- Page 10 Article No 12-1010 supply, or remove the AC cord plug. Note: Only use the power adapter supplied by Circassia with the Note: Use care not to bend the handle tube. instrument. Article No 12-1120.
- Page 11 11. Select the Settings button on the main menu. handle. This sets the remaining measurements to 1000 and expiry 12. Select Time and date. date one year from the current date. The Return button returns to Settings view without registering change. ® 000190-09 NIOX VERO User Manual US...
-
Page 12: User Interface
- blinking symbol, (j) Sound disabled, (k) Sensor status and number of remaining measurements, (l) Temperature outside of specification, (m) Humidity outside of specification, (n) Time (A) Status bar, (B) Instructive demonstration, (C) Patient ID, (D) Start measurement button ® 000190-09 NIOX VERO User Manual US... -
Page 13: Settings View
® Chapter 5 Using NIOX VERO Settings view Using NIOX VERO ® Start the instrument from power save mode ® If NIOX VERO is in standby or sleep mode simply touch the display to activate it. Register patient ID (optional) -
Page 14: Measure Feno
® Chapter 5 Using NIOX VERO Enter up to 12 characters (alpha or numeric). Note: Do not switch OFF the instrument during measurement procedure. Select the ABC-button to activate a keyboard with the alphabet. Give the breathing handle to the patient and guide the patient to provide The 123-button changes view back to the numerical keyboard. - Page 15 ® Chapter 5 Using NIOX VERO Note: The procedure is activated by inhaling air from the handle or by Exhalation with: pressing the start measurement button. Pressure correct Pressure too strong Pressure too weak Exhale until the cloud has passed the flag.
-
Page 16: Demonstration Mode
® Chapter 5 Using NIOX VERO The result is then displayed: (A) Patient ID - if applicable, (B) FeNO value in Select OK button to confirm the changes. ppb (parts per billion), (C) Measurement mode, 7. The undo button returns to the main menu without saving changes. -
Page 17: Measure Ambient No
® Chapter 5 Using NIOX VERO Measure ambient NO The progress bar is visible until the measurement is finished and the result is displayed: Ambient measurement value (in ppb), measurement Note: An ambient measurement may be requested by customer support mode, and measurement sequence number. - Page 18 Select the Patient measurements log view button. Alerts are stored in the instrument and can be viewed at any time. The alert codes are for Circassia Technical Support use. Select the Settings button on the main menu. Select Alert log button.
-
Page 19: Turn Off The Instrument
(E) Return to settings, (F) Numbers of remaining measurements on the Sensor, (G) Sensor serial number, (H) Sensor expiration date, (I) Enter Select the Return button to return to settings. configuration code (only used on request from Circassia) 5.6.5 View instrument information Turn off the instrument Detailed information about the instrument and Sensor can be viewed. -
Page 20: External Quality Control (Qc) Procedure
The external Quality Control is one of the procedures that ensures the sys- tem is operating within the specifications. ® Note: Always use a closed bag or case (NIOX VERO bag recommended) for transportation and storage of the instrument. Note: The Quality Control function must always be activated as a daily QC measurement is mandatory when the instrument is clinically used. - Page 21 ® Chapter 5 Using NIOX VERO • Non-smoker. • Acute seasonal allergy • Always attach a new patient filter for each new QC tester. • Expected stable FeNO values between 5 and 40 ppb. Select QC. • Preferably no allergies (except seasonal, see below) or asthma.
- Page 22 ® Chapter 5 Using NIOX VERO Exhale slowly through the filter while keeping the cloud within the limits 11. The progress bar is visible until the analysis phase is complete. The QC as indicated on the display (the white lines).
- Page 23 Press to return to main menu. Repeat the QC test if the positive and/or the negative control fail. If the QC ® failure persists, discontinue use of NIOX VERO and contact Circassia Technical Support. Note: The prompt to QC the device will remain if the QC measurement was performed by a non-qualified QC candidate.
- Page 24 ® Chapter 5 Using NIOX VERO Select the Settings button on the main menu. The following window is displayed: Select QC tester button. The QC qualifying results are displayed as follows. Select the Delete button for the user ID to be reset.
-
Page 25: Using Niox Vero With Niox Panel
® • When selecting an accessory for your NIOX Panel product keep in mind that an accessory not recommended by Circassia Inc may result in loss of * Needed for Bluetooth communication performance, damage to your NIOX ® Panel product, fire, electric shock, injury or damage to other property. -
Page 26: Connect To A Pc Via Usb
If the instrument is in power saving mode no connection will be established. Setup An enabled USB connection is displayed on NIOX VERO with Turn on the PC and monitor. a symbol on the status bar. Turn on the instrument. - Page 27 NIOX Panel ® Plug the USB cable into the USB port on the NIOX VERO Circassia recommends completion of the details dialog box to allow receipt of and connect it to the USB port on the PC or connect by technical data and provide better service and support functions to its custom- Bluetooth.
-
Page 28: Firmware Update
If the connection to Microsoft Azure is lost or the user has chosen to not send technical data, the cloud icon is crossed over To decide at a later stage to allow Circassia to collect data, press cancel and the dialog box will open again next time NIOX Panel is started, or click on the cloud icon in the status bar. - Page 29 Select Alert log button. (A) Alert code (for customer support purpose only), (B) Return - returns to previous view, (C) Date and time of alert, (D) Scroll list (blue), (E) Download service data (only used upon request by Circassia) 000190-09 NIOX VERO ®...
-
Page 30: Troubleshooting
The tables below provide the alert codes and recommended actions to be taken for an alert code. If the alert persists, contact your local Circassia representative or Circassia Technical Support. Measurement failed Remove any sources of disturbance... - Page 31 Continue measuring at this frequency causes condensation in the instrument and will make the instrument unusable Configuration code error for 30 minutes Only provided by Circassia upon Memory access failure request. Contact Circassia Technical support. The configuration code entered is incorrect.
- Page 32 Check the Bluetooth connection with the The self test of the instrument failed. Restart the instrument. When finished click the OK button. If alert code persists contact Circassia Technical support. USB connection error Internal hardware error Check the USB connection with the PC.
- Page 33 NO scrubber result over 10 ppb. Check that the handle cap was attached when Sensor warning instructed. Contact Circassia Technical support. Restart the QC measurement. This warning indicates that the sensor If continuously shown replace the NO may soon stop working due to battery scrubber.
- Page 34 Order a new Sensor. This alert is visible when less than 10% of the It is still possible to view measurements measurements remain or less than 2 stored in the instrument memory. weeks until expiry date. Press OK to acknowledge. 000190-09 NIOX VERO ® User Manual US...
-
Page 35: Preventive Care
The breathing handle and the instrument can not be cleaned with an aerosol. • Do not use disinfectants or wipes containing alcohol, these might permanently damage the sensor and instrument. • Do not use spray detergents. ® 000190-09 NIOX VERO User Manual US... -
Page 36: Change Disposables
(see page 40). If any item is missing or damaged, contact your local Circassia representa- tive or Circassia Pharmaceuticals Inc. Change disposables (A) Breathing handle symbol, (B) Remaining number of measurements, (C) Expiration date, (D) Breathing handle reset button, (E) Return button 8.2.1... - Page 37 Chapter 8 Preventive care 8.2.2 Exchange of NIOX VERO ® Sensor Remove the used handle from the instrument by pushing the socket Turn off the instrument. into the device and gently pulling out Open the compartment on the back of the instrument using a screwdriver.
-
Page 38: Operational Life-Time
® Patient filter Open the compartment lid (see previous section). The shelf life of the NIOX VERO Patient Filter in its unopened primary pack- age is 3 years from manufacturing date. Remove the old battery and insert a new battery. -
Page 39: Return Shipments
NIOX VERO. Normally a maximum of 10 exhalations/hour can be performed with NIOX VERO during continuous use. However, it is possible to perform 20 exhalations in one hour if the instrument is paused for a minimum of 30 minutes prior to the next session of exhalations. -
Page 40: Substances Disturbing Feno Measurement
Despite the depressant effect of smoking, smokers shown to transiently reduce exhaled NO levels, it is recommended that with asthma still have raised FeNO. Subjects should not smoke in the NO measurement be performed before spirometry. The same stipulation ® 000190-09 NIOX VERO User Manual US... -
Page 41: Electromagnetic Immunity
® NIOX VERO exercise. It would seem prudent to avoid strenuous exercise for 1 hour before the measurement. WARNING! NIOX VERO ® should not be used adjacent to or stacked with oth- • Ethnic differences in ‘healthy’ FeNO levels have been observed. In er equipment. -
Page 42: Emission Of Electromagnetic Energy
NIOX VERO. Normally a maximum of 10 exhalations/hour can be performed with NIOX VERO during continuous use. However, it is possible to perform 20 ex- halations in one hour if the instrument is paused for a minimum of 30 minutes prior to the next session of exhalations. -
Page 43: 10 Reference Information
Warning - temperature is not within operating conditions range 10.1.2 Main menu buttons Audio - mute Warning - humidity is not within operating conditions range Demo Settings Instrument connected via Time Patient ID ® 000190-09 NIOX VERO User Manual US... -
Page 44: Symbol Explanation
The Device includes a Radio Frequency (RF) transmitter Cloud - goal reached (Bluetooth) Cloud - warning pressure too high or too low NRTL-listed Prescription use only 10.3 Symbol explanation Responsible manufacturer The product meets the requirements of applicable European directive ® 000190-09 NIOX VERO User Manual US... -
Page 45: 11 Technical Data
< 10 ppb for values ≤ 50 ppb, < 20% for values > 50 ppb. Expressed as the < 65 dBA, at a distance of 1 m difference between a NIOX MINO ® FeNO value and the corresponding FeNO ® value measured with NIOX VERO instrument from Circassia. 11.4 Exhaled NO - performance data 11.9 Inhalation parameters The instrument is verified to fulfill the specified performance under the tem- perature range within 50 to 95 °F, relative humidity range of 20-80% and pres-... -
Page 46: Exhalation Parameters
11.14.1 R&TTE Directive 11.11 Essential performance Hereby, Circassia AB, declares that this NIOX VERO is in compliance with Essential performance for NIOX VERO consists of the essential requirements and other relevant provisions of Directive 1999/5/ The measurement of the NO concentration of exhaled human breath The control of exhaled breath for Asthma management according to 11.14.2 IC... -
Page 47: Rechargeable Battery Capacity
11.15 Rechargeable battery capacity Changes or modifications not expressly approved by the party responsible for Only use the power adapter or USB cable supplied by Circassia to charge the compliance could void the user’s authority to operate the equipment. battery. -
Page 48: Niox Vero
• Relative humidity range: 20% to 80%, non condensing. keep in mind that an accessory not recommended by Circassia may result in • Temperature range: 50 to 95 °F loss of performance, damage to your NIOX VERO product, fire, electric •... -
Page 49: Medical Device Reporting (Mdr)
Disposable filter to be changed for each patient. ous injury. Manufacturers of medical devices are obliged to report adverse incidents to the FDA. Any user of Circassia's products, who experience an ad- verse event related to the product, must therefore immediately report this to Circassia Inc. -
Page 50: Guidance And Manufacturer's Declaration - Electromagnetic Immunity And Electromagnetic Emissions
Guidance and manufacturer's declaration - electromagnetic immunity ® The NIOX VERO is intended for use in the electromagnetic environment specified below. The customer or the user of the NIOX VERO should assure that it is used in such an environment. Immunity test Compliance... - Page 51 95% dip in typical commercial or hospital equipment should be used not closer to interruptions U_T) for 0,5 environment. any part of the NIOX VERO, including and voltage cycle cables, than the recommended ® If the user of the NIOX VERO...
- Page 52 NIOX VERO can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF ® communications equipment (transmitters) and the NIOX VERO recommended below, according to the maximum output power of the communications equipment. 000190-09 NIOX VERO ®...
- Page 53 ® The NIOX VERO is intended for use in the electromagnetic environment specified below. ® The customer or the user of the NIOX VERO should assure that it is used in such an environment Emission test Compliance Electromagnetic environment - guidance ®...
- Page 55 Patents: Circassia’s NIOX products are protected by a number of patents in the US, Europe and a range of other countries. Circassia AB, an ISO 13485 certified company Circassia Pharmaceuticals Inc, 5151 McCrimmon Parkway, Morrisville, Suite 260, NC 27560, USA Phone: +1 (866) 275-6469, Fax: +1 (877) 630-6469, E-mail: service.us@circassia.com...


Need help?
Do you have a question about the NIOX VERO and is the answer not in the manual?
Questions and answers