Table of Contents
Advertisement
Quick Links
The Universal XactTrace system is used during sleep studies to acquire and
transfer data related to thoracic and abdominal respiratory effort to a compatible
sleep recorder.
This document describes how to use the Universal XactTrace system and
includes information on intended use, warnings and cautions, system
components, adjusting and attaching the belts, battery status, storage, cleaning,
disposal, appropriate equipment to use with the system, and technical
specifications.
Intended Use
The Universal XactTrace system measures respiratory effort to assist in the
diagnosis of sleep disorders or sleep related respiratory disorders. The
respiratory effort signals measured are processed to provide electrical signals
suitable for the inputs of physiological recording equipment.
The intended environments are hospitals, institutions, sleep centers, sleep
clinics, or other test environments. The Universal XactTrace system is intended
for diagnostic purposes only and is not intended to be used as an apnea monitor.
Use the Universal XactTrace system only under the direction and supervision of
a physician or trained technologist.
1
Advertisement
Table of Contents

Summary of Contents for natus Universal XactTrace
- Page 1 The Universal XactTrace system is used during sleep studies to acquire and transfer data related to thoracic and abdominal respiratory effort to a compatible sleep recorder. This document describes how to use the Universal XactTrace system and includes information on intended use, warnings and cautions, system...
-
Page 2: Essential Performance
® Universal XactTrace User Instructions Essential Performance In normal operational mode, essential performance is defined as the following: • Output signals may contain electrical artifacts which are distinguishable to a medical professional and must self-recover after test. The signal can hesitate or freeze under immunity testing, as long as it recovers after stimulus is removed, with no net change in the signal. - Page 3 ® Universal XactTrace User Instructions Symbol Title Standard Standard Title of as per Symbol Explanation Reference Symbol Referenced Standard Medical devices — Symbols to be used ISO 15223-1 with information to Country of Indicates the country Symbol be supplied by the Origin of origin.
-
Page 4: Warnings And Cautions
® Universal XactTrace User Instructions Symbol Title Standard Standard Title of as per Symbol Explanation Reference Symbol Referenced Standard Hand wash only Do not tumble dry Indicates that electrical and Disposal at electronic equipment Waste Electrical and end of Directive... -
Page 5: System Components
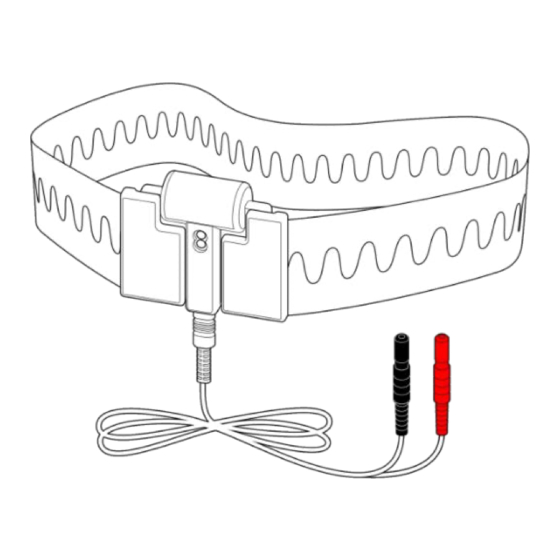
• The device has no user serviceable parts and must be serviced by Embla and authorized parties only. Warranty void if opened. Contact Natus Technical Support (Ottawa.TechSupport@natus.com) for more information. • The device is not defibrillator proof. - Page 6 ® Universal XactTrace User Instructions Belt Sensor Bipolar Cable Adjusting the Belt Size Adjust the belt by sliding the adjustment buckles together or apart to fit a patient circumference of 65–200 cm (26–78 in). To lengthen the belt: Slide the left adjustment buckle toward the center of the belt. A loop forms as shown.
- Page 7 The belts are intended to be worn over nightclothes and should fit the patient snugly without being uncomfortably tight. Avoid all unnecessary contact with moisture when using the Universal XactTrace system. To attach the XactTrace belt to the patient: Note: Do not use two thorax sensors or two abdomen sensors in the same recording.
-
Page 8: Battery Status
Note: The battery cannot be replaced. Disconnect the sensor from the belt before storing the system. Storage Proper storage extends the life of the Universal XactTrace system. To prevent damage, do the following when storing the belts and cables between studies. - Page 9 To save battery power, disconnect the sensor from the belt when not in use. Cleaning No part of the Universal XactTrace system requires sterilization. • Sensor and Cables. Wipe clean with a hospital grade cleaner that is not corrosive to plastic or metal, and then dry with a clean, dry cloth. Do not immerse the sensor in liquid and avoid contact of the cleaning solution with the connectors.
-
Page 10: Maintenance
® Universal XactTrace User Instructions Maintenance No special maintenance of the Universal XactTrace system is required. Technical Specifications Description Properties Power Battery Non replaceable Li-SOCl2 type ER14250. Nominal operation time 2000 hours (approximately 250 eight-hour studies). Environmental Temperature Operation: +5°C to +41º ºC (40°F to 106º F) Specifications Storage: -18°C to +48ºC (0°F to 120º... - Page 11 Ingress of Equipment classified as ordinary equipment Liquids regarding ingress of liquids; that is, it is not drip-proof, splash-proof or watertight. Connect the Universal XactTrace only to recording/monitoring equipment with patient-connected input channels that comply with IEC60601-1, type CF or BF.
-
Page 12: Safety Standards
Table 1 – Electromagnetic Emissions Guidance and manufacturer’s declaration – electromagnetic emissions The Universal XactTrace Belt is intended for use in the electromagnetic environment specified below. The customer or the user of the equipment should assure that it is used in such an environment. - Page 13 ® Universal XactTrace User Instructions Table 4 – Immunity Test Levels – Enclosure Port Basic EMC Immunity Test Levels – Professional Phenomenon Standard or Test Healthcare Facility Environment Method ± 8 kV contact Electrostatic Discharge IEC 61000-4-2 ± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air 3 V/m 80 MHz –...
- Page 14 ® Universal XactTrace User Instructions Table 9 – Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment IMMUNITY Test Maximum Band Distance TEST frequency Service Modulation Power (MHz) LEVEL (MHz) (V/m) Pulse 380 – TETRA modulation 18 Hz GMRS 430 –...
-
Page 15: Warranty
® Universal XactTrace User Instructions Warranty ® Embla warrants the sensor to be free of defects in materials and workmanship for one year from the date purchased. The sole liability of Embla and our distributors is limited to replacement or repair of the product at the option of Embla, with no charge for parts or labor if any part is proven to be defective in workmanship, performance, or materials during the warranty period. - Page 16 This copy of the User Manual shall be used only in accordance with the conditions of sale of Natus Medical Incorporated or its distributors. Natus Medical Incorporated makes no representations or warranties of any kind whatsoever with respect to this document. Natus Medical Incorporated disclaims all liabilities for loss or damage arising out of the possession, sale, or use of this document.












