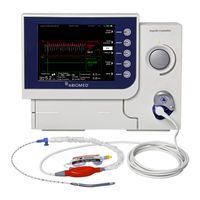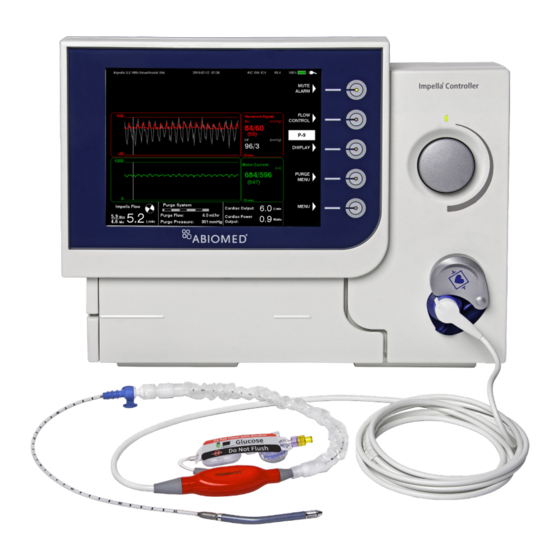
Abiomed Impella 5.5 Manuals
Manuals and User Guides for Abiomed Impella 5.5. We have 2 Abiomed Impella 5.5 manuals available for free PDF download: Instructions For Use Manual, User Manual
Abiomed Impella 5.5 Instructions For Use Manual (243 pages)
with SmartAssist. Impella 5.5
Brand: Abiomed
|
Category: Medical Equipment
|
Size: 22 MB
Table of Contents
Advertisement
Abiomed Impella 5.5 User Manual (88 pages)
Circulatory Support System with SmartAssist
Brand: Abiomed
|
Category: Medical Equipment
|
Size: 16 MB
Table of Contents
Advertisement