Advertisement
Quick Links
EXALT
Model D
™
F O R U S E W I T H T H E E X A L T C O N T R O L L E R
Single-Use Duodenoscope
M00542420, M00542421
TABLE OF CONTENTS
REUSE WARNING ................................................................... 1
DEVICE DESCRIPTION ............................................................ 1
Contents ............................................................................ 1
Operating Principle ........................................................... 1
Features and Controls ........................................................2
Figure 1. .....................................................................................2
Figures 2 and 3. Main features of the Endoscope ......................3
Figure 4. .....................................................................................4
Figure 5. .....................................................................................5
Articulation Section ...........................................................5
Materials ............................................................................5
User Information................................................................5
Model Numbers .................................................................5
Specifications .....................................................................6
Table 1. Endoscope specifications ......................................6
INTENDED USE/INDICATIONS FOR USE ................................6
Clinical Benefit Statement .................................................6
CONTRAINDICATIONS ............................................................6
WARNINGS .............................................................................6
PRECAUTIONS ....................................................................... 8
ADVERSE EVENTS ................................................................10
CONFORMANCE TO STANDARDS ........................................10
Essential Performance Statement ...................................10
HOW SUPPLIED ....................................................................10
Device Details ..................................................................10
Handling and Storage ......................................................10
DEVICE COMPATIBILITY .......................................................10
OPERATIONAL INSTRUCTIONS ............................................10
Unpack and Inspect the Endoscope.................................10
Attaching the suction and air/water valves ......................11
Attaching the biopsy valves .............................................11
Connect the Endoscope to the Controller and
check the Image ...............................................................11
Figure 6. Inserting the umbilicus connector into the
Controller receptacle ................................................................ 12
Attach Suction and Air/Water Tubing .............................. 12
Test Suction and Air/Water Valves................................... 12
Test Working Channel and Elevator Function .................. 12
Preparation and Insertion of the Endoscope ................... 12
Inserting an Endoscopic Accessory Device into the
Single-Use Endoscope ..................................................... 13
Locking the guidewire ..................................................... 13
Removing an Accessory Device from the Endoscope ...... 13
Removing the Endoscope from the Patient ..................... 13
Disposal ........................................................................... 14
Post-Procedure ................................................................ 14
PATIENT COUNSELING INFORMATION ................................ 14
WARRANTY .......................................................................... 14
SYMBOL DEFINITIONS ......................................................... 14
ONLY
Caution: Federal Law (USA) restricts this device to sale by or on the order
of a physician.
REUSE WARNING
For single use only. Do not reuse, reprocess or resterilize. Reuse,
reprocessing or resterilization may compromise the structural integrity
of the device and/or lead to device failure which, in turn, may result in
patient injury, illness or death. Reuse, reprocessing or resterilization may
also create a risk of contamination of the device and/or cause patient
infection or cross-infection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another. Contamination of the
device may lead to injury, illness or death of the patient.
DEVICE DESCRIPTION
Contents
The package includes:
One (1) EXALT Model D Single-Use Duodenoscope.
Ensure the package contains the components listed below.
Operating Principle
The EXALT Model D Single-Use Duodenoscope (hereafter referred to as
the Endoscope) is a sterile, single-use Endoscope that facilitates access to
the duodenum, delivery of accessories, and live video when connected to
an EXALT Controller (hereafter referred to as the Controller).
Black (K) ∆E ≤5.0
Advertisement

Summary of Contents for Boston Scientific EXALT D
- Page 1 EXALT Model D ™ F O R U S E W I T H T H E E X A L T C O N T R O L L E R Single-Use Duodenoscope M00542420, M00542421 TABLE OF CONTENTS ONLY REUSE WARNING ..............
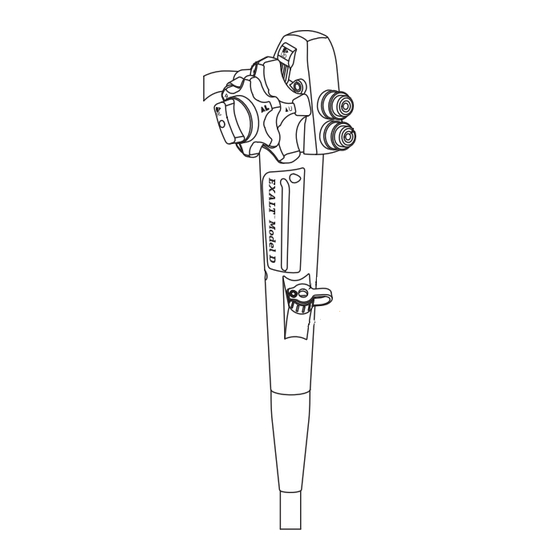
- Page 2 Features and Controls The Endoscope includes the following features and controls: 1. Handle 2. Insertion Portion 3. Insertion Tube 4. Articulation Section 5. Distal Tip 6. Maximum Insertion Mark 7. Umbilicus Section 8. Umbilicus 9. Umbilicus Connector Figure 1. Black (K) ∆E ≤5.0...
- Page 3 10. Up/Down Articulation Control Knob 11. Up/Down Lock 12. Left/Right Articulation Control Knob 13. Left/Right Lock 14. Elevator Lever 15. Image Capture Button 16. Suction Valve* 17. Air/Water Valve* 18. Biopsy Port 19. Biopsy Valve* *not included Figures 2 and 3. Main features of the Endoscope •...
- Page 4 • Suction valve: Depressing this valve activates suction. Refer to the instructions for use for the Orca Suction Valve for further description and intended use. The Endoscope shall only be used in conjunction with the Orca sterile single- use suction valve (not included). •...
- Page 5 24. Suction Connector 25. Air/Water Connector Figure 5. The Endoscope works in conjunction with the Controller. • Two knobs and one lever on the handle assist in navigation, delivery and removal of accessory devices: o UP/DOWN articulation control knob manipulates the bending section in the UP/DOWN directions o LEFT/RIGHT articulation control knob manipulates the bending section in the LEFT/RIGHT directions o Elevator lever controls the articulation of the elevator •...
- Page 6 Specifications The Endoscope specifications are summarized in Table 1. Table 1. Endoscope specifications Specifications Value Direction of view Backward side viewing 5° Field of view 108° Up: 120° Down: 90° Articulation Angle Left: 90° Right 110° Maximum elevator articulation angle (with guidewire) ≥80°...
- Page 7 • Before use, inspect the outer surface of the portion of the Endoscope which is intended to be inserted into a patient or used during the procedure. Do not use an Endoscope that has rough surfaces, sharp edges or protrusions which may cause patient injury such as perforation or tissue damage. Cut, burned or damaged Endoscope insulation may cause unsafe currents in either patient or user.
- Page 8 • During scope removal from the patient, failure to manually return the articulation control knobs to the neutral position or put the elevator in the down position may cause patient injury such as perforation, bleeding, or tissue damage. The articulation control knobs do not naturally return to neutral and must be manually rotated to neutral.
- Page 9 • Do not apply lubricant to the distal tip. It may contact the lens and damage the lens or render the Endoscope image unusable. • Damaging the face of the umbilicus connector may result in no visualization or an unexpected loss of visualization. Handle the connector with care and inspect the face of the umbilicus connector for damage before use.
- Page 10 (Olympus / FujiFilm G5 Series Brand Endoscope Compatible), the Seal Biopsy Valve, and Hydra Water Bottle Caps. Additionally, the Endoscope is compatible with Boston Scientific accessory devices that are compatible with a 4.2 mm working channel, have a minimum working length of 180 cm and (for energized devices) have a Maximum Accessory Voltage Rating: 3800 V peak (7600 V peak-to-peak).
- Page 11 4. Inspect the entire surface of the insertion portion visually and tactilely. Examine the handle, insertion tube, and bending section. Ensure no components are loose or broken. During inspection, do not touch the lens surface. If you observe any loose or broken components, do not use the Endoscope. 5.
- Page 12 Figure 6. Inserting the umbilicus connector into the Controller receptacle Attach Suction and Air/Water Tubing 1. Connect the air/water tubing of the Hydra Water Bottle Cap set to the air/ water connector of the Endoscope. Refer to the instructions for use for the Hydra Water Bottle Cap for proper inspection and connection. Be sure to follow all instructions and consider all warnings and precautions.
- Page 13 and lower esophageal sphincters or when the endoscopic view is obstructed, adjust the position of the tip of the Endoscope as needed, and advance slowly. 3. Manipulate the articulation control knobs as necessary under visualization to navigate the Endoscope to the desired location in the digestive tract.
- Page 14 Instructions for Use (IFU), pertaining to the patient. WARRANTY For device warranty information, visit (www.bostonscientific.com/warranty). The following are trademarks of Boston Scientific Corporation or its affiliates: EXALT, Hydra, Orca, Seal. All other trademarks are the property of their respective owners. SYMBOL DEFINITIONS Commonly used medical device symbols that appear on the labeling are defined at www.bostonscientific.com/SymbolsGlossary.
- Page 15 Ballybrit Business Park Galway IRELAND Boston Scientific Corporation 300 Boston Scientific Way Marlborough, MA 01752 USA USA Customer Service +1-888-272-1001 www.bostonscientific.com © 2024 Boston Scientific Corporation or its affiliates. All rights reserved. 2024-02 < > 51790015-01 Black (K) ∆E ≤5.0...

Need help?
Do you have a question about the EXALT D and is the answer not in the manual?
Questions and answers