Advertisement
Quick Links
Advertisement

Summary of Contents for Électronique du Mazet I-Press 10C
- Page 1 Page 1 on 31 MEG009XN102-A7 DATE 2023/01 User's manual I-Press 10C Pressure Therapy Device...
- Page 2 Page 2 on 31 MEG009XN102-A7 DATE 2023/01 Instructions for use & Technical description Please read these instructions carefully before using your new device! This manual is an integral part of the device and must be kept until it is destroyed. This equipment has been designed and manufactured for therapeutic use.
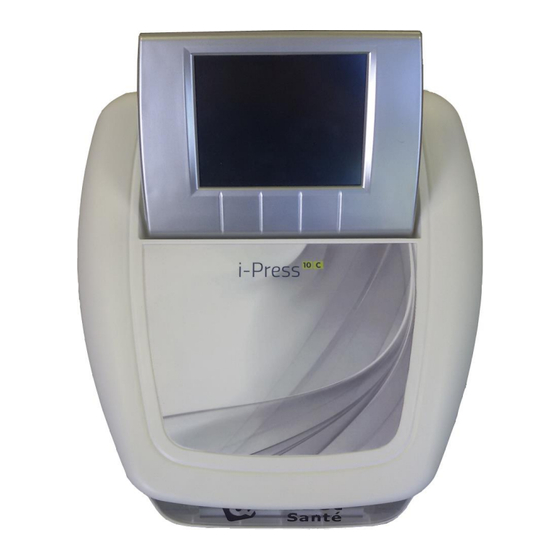
- Page 3 MEG009XN102-A7 DATE 2023/01 1 Presentation of the device The i-Press 10C is a pressure therapy device that helps in the practice of physical therapy, to treat venous disorders and lymphedema. It can also be used for wellness programs. The computerized technology used in the i-Press 10C allows for ease of use and easy menu navigation.
- Page 4 Page 4 on 31 MEG009XN102-A7 DATE 2023/01 Table of Contents : Presentation of the device ....................3 Description and technical information ................5 Symbols used ....................... 6 Technical data ......................7 2.2.1 General characteristics ........................7 2.2.2 Technical data of the device......................7 2.2.3 Accessories ............................
- Page 5 2 Description and technical information -This Operation and Maintenance Manual is published to assist you in getting started with your i-Press 10C from the initial acceptance phase, through commissioning, and on to subsequent operation and maintenance. If you have any difficulty in understanding this manual, contact the manufacturer, Électronique du Mazet, your dealer or distributor.
- Page 6 Page 6 on 31 MEG009XN102-A7 DATE 2023/01 2.1 Symbols used Warning: this logo draws your attention to a specific point Operating Instructions: This logo informs you that the operating instructions must be read for safe use of the device Type B applied part: applied part in contact with the patient and which can be connected to the ground.
- Page 7 Page 7 on 31 MEG009XN102-A7 DATE 2023/01 2.2 Technical specifications 2.2.1 General characteristics • Operating temperature: 0°C to 40°C. • Storage temperature: -40°C to 70°C. • Operating relative humidity: 30% to 75%. • Operating altitude: < 2000 meters • Operating pressure: between 80 and 110 kPa 2.2.2 Technical characteristics of the device •...
- Page 8 Page 8 on 31 MEG009XN102-A7 DATE 2023/01 2.2.3 Accessories This device is delivered with the following accessories as standard: -1 Power cord -1 Pair of 10 cell boots -1 User's Manual -1 Clinical Guide The optional accessories available are : - 7-cell sleeve - 7-cell abdominal belt - Pair of 10-cell hip braces...
- Page 9 Page 9 on 31 MEG009XN102-A7 DATE 2023/01 2.3 Nameplate label The information and characteristics are reported on the back of each device on a label 2.3.1 Device identification labels 230VAC 50VA 50Hz Rating T2AH-250V 2x size 5x20 Type B 2.3.2 Accessory identification labels xxxxxx-xxxx Boot 10P MEG009KP513A0...
- Page 10 Page 10 on 31 MEG009XN102-A7 DATE 2023/01 2.4 Warnings CAUTION: Install the unit on a flat, stable surface. Do not obstruct the ventilation openings (no objects closer than 4cm). CAUTION: Power strips must not be placed on the floor. No other electrical appliances or power strips should be connected to the power strip.
- Page 11 3 Installation of the device Open the carton, remove the accessories and the i-Press 10C Remove the light plastic wrappings that cover the unit. Check the contents of the box against the packing list included with the documentation.
- Page 12 Page 12 on 31 MEG009XN102-A7 DATE 2023/01 -Connect the power cord -Connect the accessories: Lock (red) on the left, position the connector by respecting the coding. Lock -Flip the switch : by sliding the lock to the right. Position 0: Off -Disconnect accessories: Slide the Lock to the left to Position 1: On release the connector...
- Page 13 Page 13 on 31 MEG009XN102-A7 DATE 2023/01 4.1.2 Use of the touch screen The lists of choices displayed on the screen and the validations as well as the navigation in the menus are indicated by "action" buttons on the touch screen. To access the desired function, press in the indicated area.
- Page 14 Page 14 on 31 MEG009XN102-A7 DATE 2023/01 All the menus accessible afterwards will be equipped with a button allowing the return to the main menu (placed at the top left)
- Page 15 Page 15 on 31 MEG009XN102-A7 DATE 2023/01 4.2 Choice of treatment 4.2.1 From the last treatment performed To select the last treatment performed, press the screen at the bottom left when the start page is displayed. 4.2.2 From u diagnosis Selecting the pathology to be treated, by pressing the corresponding action button, allows access to the §6.3 Parameter modification menu.
- Page 16 Page 16 on 31 MEG009XN102-A7 DATE 2023/01 4.3 Modification of parameters When accessing the treatment, and before starting it, it is possible to modify the "Time" (treatment) and "Pressure" (in the cells) parameters by pressing the "+" or "- " key of the one you wish to set. 4.4 Run a treatment 4.4.1 Start the treatment Choose the treatment category you want.
- Page 17 Page 17 on 31 MEG009XN102-A7 DATE 2023/01 This icon gives access to the treatment settings: -Working time from 0 to 15 seconds (keeping the cell pressurized) -Rest time of 4 to 15 seconds (Deflation between 2 inflations) - Number of accessories: (setting available with leg programs) Default set to 2.
- Page 18 Page 18 on 31 MEG009XN102-A7 DATE 2023/01 4.4.3 End of treatment End of treatment The end of the treatment is signaled by a succession of beeps. You can stop the active deflation to return to the general menu 4.5 Saving a treatment After stopping, at the end of a treatment or before starting the treatment, the user has the possibility to save the parameters of his treatment in one of the programs of the personalized database (see §...
- Page 19 Page 19 on 31 MEG009XN102-A7 DATE 2023/01 4.6 Technical Information, Configuration & Settings This screen provides access to technical information about the equipment, menu language selection, screen brightness adjustment, sound information selection and testing. You will then have access to the following menu: •...
- Page 20 Page 20 on 31 MEG009XN102-A7 DATE 2023/01 • Reserved access: allows you to launch a self-diagnosis of the device using a code that will be communicated to you by our after-sales service (Information button) in the event of a failure or malfunction of the device.
- Page 21 The device is intended for all adults over 18 years old, regardless of gender. 5.2 Expected performance The i-Press 10C is a pressotherapy device that helps in the treatment of venous and lymphatic pathologies by performing mechanical lymphatic drainage. This technique allows to promote the circulation known as "return". Indeed, the interest of lymphatic drainage is no longer to be demonstrated.
- Page 22 Page 22 on 31 MEG009XN102-A7 DATE 2023/01 5.3 Major contraindications This device must not be used in the following cases: - Emboligenic deep vein thrombosis - Untreated heart failure, Severe arterial disease - Skin infection (Erespele, urticaria, ...) - Sensitivity disorder - Severe renal failure - Febrile state (fever, ...) - Lymphangitis...
- Page 23 DATE 2023/01 6 Maintenance, upkeep The I-press 10C is designed to last for 5 years. To ensure that the performance of the device is maintained throughout its life, it is necessary to have the device checked by Électronique du Mazet technicians every 2 years.
- Page 24 Page 24 on 31 MEG009XN102-A7 DATE 2023/01 7 Malfunction If you notice a malfunction that is not commented on in the documents accompanying the device (see below), please inform your distributor or the manufacturer. In the case of a shipment of the device, please observe the following instructions: ▪...
- Page 25 Page 25 on 31 MEG009XN102-A7 DATE 2023/01 Possible operating anomalies: Description of the Possible causes Actions anomaly Screen off Problem of the electrical Check the power connection network Starting up the device Check the position of the on/off button (position I) Fuses out of order Check and change the fuses Other cause...
- Page 26 Page 26 on 31 MEG009XN102-A7 DATE 2023/01 8 After-sales service and warranty This device is warranted by your supplier under the conditions specified in this document, provided that: ◼ Only accessories supplied by Électronique du Mazet or its distributors should be used. ◼...
- Page 27 9.2 Electronics Should the I-Press 10C device fail or become unusable, please return it to the manufacturer or drop it off at a Recylum collection point. Indeed, as part of its commitment to the environment, Électronique du Mazet finances the recycling network Récylum dedicated to WEEE Pro, which takes back...
- Page 28 Page 28 on 31 MEG009XN102-A7 DATE 2023/01 11 CE declaration ÉLECTRONIQUE DU MAZET can provide the CE declaration for this device on request. The first affixing of the medical CE on this device took place on 14/10/2016 12 Manufacturer Électronique du Mazet is a company located in the heart of the Massif Central. Originally a simple manufacturer of electronic cards, over the years it has developed its own brand of medical equipment, mainly for physiotherapy.
- Page 29 Page 29 on 31 MEG009XN102-A7 DATE 2023/01 13 EMC compliance table EMC compliance according to IEC / EN 60601-1-2 (2014) The device is intended for use in the electromagnetic environment specified below. The customer or user of the device should ensure that it is used in such an environment. Emissions test Standard Compliance...
- Page 30 Page 30 on 31 MEG009XN102-A7 DATE 2023/01 calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance d = 1.67.√P Conducted RF 3 Vrms 3 Vrms d = 1.67.√P 80MHz-800MHz disturbances 150kHz-80MHz d = 2.33.√P IEC 61000-4-6 3V/m 3V/m 800MHz-2.5GHz...
- Page 31 Page 31 on 31 MEG009XN102-A7 DATE 2023/01 ELECTRONIQUE DU MAZET ZA ROUTE DE TENCE 43520 LE MAZET SAINT VOY Tél : +33 4 71 65 02 16 Mail : sav@electroniquedumazet.com Your dealer / distributor :...


Need help?
Do you have a question about the I-Press 10C and is the answer not in the manual?
Questions and answers