Table of Contents
Advertisement
Quick Links
Applicable Product Families:
Dabir Surgical™ System (Facility Use Only)
Applicable Model Numbers:
Controllers & Accessories (CA-XXXX)
Surfaces (DA-XXXXXX-XX-XX)
Controller
Surfaces
MD
Operations / Service Technical Manual
NOTE: Electronic copies available at
www.agilitihealth.com/dabir
Hose Assembly
DOC# R01-0006-00004-QA
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for DABIR Surgical CA-1001
- Page 1 Operations / Service Technical Manual NOTE: Electronic copies available at www.agilitihealth.com/dabir Applicable Product Families: Dabir Surgical™ System (Facility Use Only) Applicable Model Numbers: Controllers & Accessories (CA-XXXX) Surfaces (DA-XXXXXX-XX-XX) Controller Hose Assembly Surfaces DOC# R01-0006-00004-QA...
-
Page 2: Table Of Contents
Important Before You Start Safety Warnings Labels and Descriptions About this Manual Indications for Use Contraindications About the Dabir System How it Works Controller, Hose Assembly and Accessories Controller Pre-Assembly System Installation - Controller System Installation - Surfaces System Installation - Connections... -
Page 3: Important Before You Start
1.1 Important Before You Start Please read the “Instructions-for-Use” device manual carefully and completely before using Dabir products. Failure to do so may result in decreased performance or product failure. 1.2 Safety Warnings • WARNING: To avoid patient injury, do not place the installed Surface before powering the Controller "ON". -
Page 4: Labels And Descriptions
1.3 Labels and Descriptions The Symbols below appear on the Controller, Surfaces, Hose Assembly, Accessories and/or packaging. Label Description Application UL Mark Controller label ANSI/AAMI ES60601-1 AMD (2012), “Medical Electrical Equipment - Part 1: General Requirements for Basic Safety and Essential Performance, Amendment 1”. - Page 5 1.3 Labels and Descriptions - Continued The Symbols below appear on the Controller, Surfaces, Hose Assembly, Accessories and/or packaging. Label Description Application Follow instructions for use Controller label, Surface labels, Hose Assembly label Defibrillation-proof Type BF Applied Part Controller label Indicates a defibrillation-proof type BF applied part complying with IEC 60601-1.
- Page 6 1.3 Labels and Descriptions - Continued The Symbols below appear on the Controller, Surfaces, Hose Assembly, Accessories and/or packaging. Label Description Application Serial Number Box labels, Surface label, Se- Indicates the manufacturer’s serial number so that a rial ID labels, Surface labels specific medical device can be identified.
-
Page 7: About This Manual
NOTE: All labels are to be read from 0.5m. 1.4 About this Manual This manual is your introduction to the Dabir Surfaces Patient Support System. Use it to assure proper installation of the Controller, Hose Assembly and Surface. Also keep it as a reference for day-to-day operation, annual service and maintenance. -
Page 8: About The Dabir System
Low Profile & Self Contouring Dabir Micropressure Surfaces are designed to be thin and flexible so that they do NOT impact the overall height of the bed mattress. They also contour to the shape of the individual patient as they immerse into a mattress, which provides the broad pressure redistribution support needed beneath Dabir. -
Page 9: Controller, Hose Assembly And Accessories
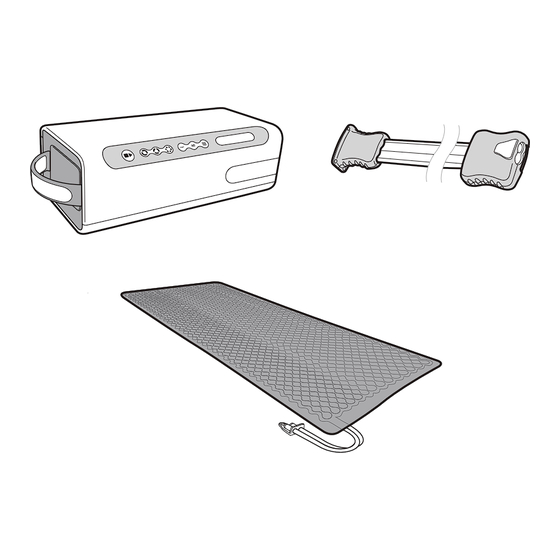
2.0 Controller, Hose Assembly, Surfaces and Accessories Controller User Interface Panel Transport Handle Hose Assembly Connector (Male / Orange) Connector (Female / White) Surfaces: (Multi-Patient Use) Connectors (Male) Vent Accessories See individual component packaging for Accessory related instructions. DOC# R01-0006-00004-QA... -
Page 10: Controller Pre-Assembly
3.1 Controller Pre-Assembly Place Controller on a flat, stable surface away from any edges to prevent fall damage. Insert Hose Assembly Connector (Male) into the female end of the Controller until fully seated. Insert the four (4) provided screws and tighten as shown below. (#1 Philips screwdriver included) Insert Power Cord into the receptacle shown below. -
Page 11: System Installation - Controller
Place Controller in a safe, stable location near a wall power outlet and approximately 3 feet away from the patient support surface . Please refer to Dabir Accessories for alternative mounting options. Route Power Cord and Hose Assembly such that it does not present a trip hazard. -
Page 12: System Installation - Surfaces
3.3 System Installation - Surfaces 1. Place Dabir Surface flat onto pad or mattress with “THIS SIDE UP” facing up (gray side down) and secure optional corner straps when applicable. (Surface should lay flat.) Hose routing is preferred at the foot end. -
Page 13: System Installation - Connections
3.4 System Installation - Connections 1. Connect Hose and Surface with Controller “PAUSED” or powered “OFF”. Orange Release Button Surface Connector (Male) Hose Connector (White / Female) Proper connection shown here. CAUTION: To avoid damage, do not drop Hose Assembly to floor after connecting. -
Page 14: Operations And Settings
Step 2: Touch “START/STOP” Key to activate Surface cycle. Note: The Dabir Auto-start feature will activate the system within 30 seconds of main power "ON" or within 10 minutes of being stopped during normal operation. The "START/STOP" key is still functional at any time while the Device is powered "ON". -
Page 15: Interrupting, Rapid Deflate And Resuming Operation
5 Interrupting, Rapid Deflate, and Resuming Operation Interrupting or Rapid Deflate Options: Main Power “OFF” Toggle the main power switch to the “OFF” position. NOTE: Turning power “OFF” will automatically deflate the Surface and reset Cycle Speed and Firmness to default settings. CAUTION: Power “OFF”... -
Page 16: Information Center: Alert Codes
Surface and start the unit again. (See next page for further diagnosis codes.) REPLACE OVERLAY All Dabir Surfaces include smart electronics which instruct the Controller when its preprogrammed usage life has expired. Simply replace the overlay when prompted. CAUTION: Once Surface life expires, power interrupt will force surface replacement. Depending on the model, pausing therapy or disconnecting the surface may also force surface replacement. -
Page 17: Alert Buzzer
6 Information Center: Alert Codes - Continued Information Center Indicator Alert Buzzer Meaning Check Frequency: 2 kHz Controller improperly communicating with the Connections Surface. Check Hose to Surface connection. The 2 sec "ON" after touching light will clear when proper connection is made, Continuous START/STOP key, returning function to normal operation. -
Page 18: Cleaning Procedures
Approved & Unapproved Cleaners Please visit: www.agilitihealth.com/dabir. NOTE: Test results have shown that the above "unapproved cleaners" will reduce Dabir product life with repeated use. Surface Disposal Please follow Section 7 Cleaning Procedures prior to disposal or recycling per hospital standard procedure. -
Page 19: Preventative Maintenance / Service
8 Preventative Maintenance / Service The Dabir Surfaces Patient Support System Routinely check all electrical connections, Power Cord and Hose Assembly for signs of wear or damage and replace if necessary with certified parts. For any non-serviceable damage, please contact the manufacturer. - Page 20 Replaceable Components: Controller, Power Cord, Hose Assembly, Screws System Performance Check (POWER CONNECTED) REQUIRED: ASSEMBLED CONTROLLER and a DABIR SURFACE (Any model listed: dabir-surfaces.com/models) Set-up Instructions: Follow the procedure defined in Section 3. (Surface does NOT require surgical table, stretcher or bed installation for performance check. No tools required.) Perform the following system checks to confirm basic function: 1.
-
Page 21: Settings Function
8 Preventative Maintenance / Service - Continued System Performance Check (continued) 2. AUTOSTART FEATURE: • Place the controller on a safe, stable work location and connect the surface. • Confirm that the unit is plugged in and powered "OFF". • Power the unit "ON". (Reference Section 4, Step 1) System start-up mode should begin with flashing lights and audible tones. -
Page 22: Notes / Comments
8 Preventative Maintenance / Service - Continued System Mechanical Diagram (Controller Only) Primary Components List: 1. Top Case Cover Assembly (1) 2. Pump Mounting Screw (4) 3. Air Inlet Screen (1) 4. Pump Assembly (1) 5. Pump Isolators (2) 6. Cage Mounting Screws (4) 7. - Page 23 8 Preventative Maintenance / Service - Continued System Electrical Schematic (Controller Assembly) DOC# R01-0006-00004-QA...
-
Page 24: Troubleshooting
Power “OFF” the Controller. Controller. Contact manufacturer. NOTE: If fuse is blown - contact Dabir technical support for service. (DO NOT REPLACE) How to reach us: Dabir Surfaces maintains regular business hours from 9:00 a.m. to 5:00 p.m. Eastern time. -
Page 25: Specification Sheets
10 Specification Sheets Controller Specifications Model: CA-1001 (Controller Only), CA-1000 (Starter Kit) Supply Voltage: 100-240 VAC Supply Frequency: 50-60 Hz Power Input: 170 VA Max Size: 356 x 178 x 127 mm (14 x 7 x 5 in.) Weight: 3.95 kg (8.7 lb) Case Material: Plastic Fuse Ratings:... -
Page 26: Accessory Specifications
10 Specification Sheets - Continued Accessory Specifications Replacement Hose Assembly Model: CA-9001 Family Weight: 0.73kg (1.6lb) Input: 5VDC 250mA RF Transmitter Separate collection for electronic waste Replacement Power Cord Model: CA-90B2 Family Weight: 0.34 kg (0.75 lb.) Length: 10’ Wall Plug: NEMA 5-15P 3P Hospital Grade Green Dot Controller Plug: IEC 60320-C13... - Page 27 10 Specification Sheets - Continued Model CA-1001 & CA-1000 Manufacturer’s Declaration- Electromagnetic Immunity The Model CA-1001 or CA-1000 system is intended for use in the electromagnetic environment specified below. The customer or the user of the Model CA-1001 & CA-1000 system should assure that it is used in such an environment.
- Page 28 10 Specification Sheets - Continued Model CA-1001 & CA-1000 Manufacturer’s Declaration- Electromagnetic Immunity The Model CA-1001 or CA-1000 system is intended for use in the electromagnetic environment speci- fied below. The customer or the user of the Model CA-1001 or CA-1000 system should assure that it is used in such an environment.
- Page 29 10 Specification Sheets - Continued Model CA-1001 & CA-1000 Manufacturer’s Declaration-Electromagnetic Emissions The Model CA-1001 or CA-1000 system is intended for use in the electromagnetic environment specified below. The customer or the user of the Model CA-1001 or CA-1000 system should assure that it is used in such an environment.
- Page 30 10 Specification Sheets - Continued Changes or modifications not expressly approved by Dabir Surfaces, lnc. could void the user’s authority to operate the equipment. This device complies with Part 15 of the FCC Rules and with Industry Canada license-exempt RSS standard(s). Operation is subject to the following two conditions: (1) this device may not cause inter- ference, and (2) this device must accept any interference, including interference that may cause undesired operation of the device.
-
Page 31: Limited Product Warranty
Limited Product Warranty D-L00-001 Rev. 5 Dabir Surgical™ System, Dabir Patient Care Plus™ System PLEASE READ THESE WARRANTY TERMS AND CONDITIONS CAREFULLY BEFORE USING YOUR AGILITI PRODUCT. BY USING THE PRODUCT, YOU ARE CONSENTING TO BE BOUND BY THE FOLLOWING WARRANTY TERMS AND CONDITIONS. -
Page 32: Exclusions And Limitations
substitute materials if original materials are no longer available. — If Sizewise Manufacturing determines the product or part that Buyer has requested warranty services on are not covered by the warranty for any reason including, without limitation, because it is outside of the warranty period, excluded from the warranty, or the warranty is void, Buyer shall pay for the repair or replacement services, including parts and labor performed by Sizewise Manufacturing at Sizewise Manufacturing’s prevailing time and material rates plus freight and delivery. -
Page 33: Exclusive Remedies
CLAIM, OR REMEDY FOR LOSS OF OR DAMAGE TO ANY PRODUCT OR PART(S). This disclaimer and release shall apply even if the express warranty set forth above fails of its essential purpose. SOME STATES DO NOT ALLOW DISCLAIMERS OF IMPLIED WARRANTIES, SO THIS DISCLAIMEER MAY NOT APPLY TO YOU. -
Page 34: Contact Information
Eden Prairie, MN 55344 Tel: +1(800)847-7368 Manufacturer Raye's, Inc, d/b/a Sizewise Manufacturing 252 Mariah Circle Corona, CA 92879 Tel: +1(800)814-9389 Technical and Warranty Support agilitihealth.com/contact Tel: +1(888)208-4599 Dabir® and Dabir Surfaces® are registered trademarks of Agiliti Health, Inc DOC# R01-0006-00004-PA...

Need help?
Do you have a question about the Surgical CA-1001 and is the answer not in the manual?
Questions and answers