Summary of Contents for Medi-Plinth Physio+ 2 Section
- Page 1 Part Number: SD01006 Iss 01 11/2023 Physio+ 2 & 3 Section Plinths Instructions for use PHYS+02E/02H, PHYS+03E/03H...
-
Page 2: Table Of Contents
Part Number: SD01006 Iss 01 11/2023 Contents DEVICE DESCRIPTION ..................... 3 INTENDED USE ......................3 INDICATIONS FOR USE .................... 3 INTENDED USER AND USER TRAINING ..............3 PATIENT TARGET GROUP(s) ................... 3 PERFORMANCE CHARACTERISTICS ..............3 EXPECTED CLINICAL BENEFIT ................4 CONTRAINDICATION .................... -
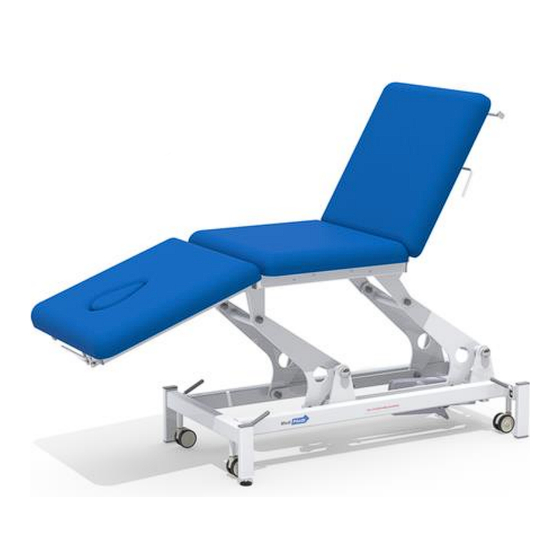
Page 3: Device Description
1. DEVICE DESCRIPTION The Medi-Plinth 2/3 Section Physio+ Plinths consists of a platform mounted on a stable frame designed to support a maximum weight of 260 kg (225kg hydraulic versions) and fitted with either a hydraulic or electric elevation system. -
Page 4: Expected Clinical Benefit
Before using any plinth/couch, please familiarize any operator with this user manual. All product IFU’s can be found on our website: www.medi-plinth.co.uk/IFU. The QR code located next to the serial number on the product will directly link you to the IFU also. Should you require a printed hardcopy of the IFU, please email sales@medi-plinth.co.uk... - Page 5 • Other than for routine daily procedures listed in the troubleshooting in this manual, any maintenance or repairs to Medi-Plinth products must be carried out by an authorised, competent and Medi-Plinth approved engineer who has been trained and certified by Medi-Plinth Equipment Ltd.
-
Page 6: Potential Adverse Effects
Interconnecting cables must remain plugged in during cleaning to prevent water ingress. • Only original and approved spare parts must be used with Medi-Plinth plinths/couches – failure to use original or approved spares will invalidate the warranty and may cause injury or damage. -
Page 7: Adjustable Foot & Wheel System
Part Number: SD01006 Iss 01 11/2023 • Before using the plinth, test every function and check that it is in perfect conditions of use. ADJUSTABLE FOOT & WHEEL SYSTEM The 2&3 section Physio+ Plinths are equipped with an adjustable foot and retractable wheel system. - Page 8 Part Number: SD01006 Iss 01 11/2023 Ensure that the mains lead, and hand switch/foot switch cables are not tangled around the frame of the plinth and fit the mains plug into a suitable mains supply socket. The plinth can be raised and lowered by pushing the appropriate buttons on your chosen control handset or foot switch, if fitted.
-
Page 9: Operating Environment
Part Number: SD01006 Iss 01 11/2023 Raise Lower 13. OPERATING ENVIRONMENT Operation of this equipment must be within the following ambient condition: Temperature + 5 °C to + 40 Relative humidity 20% to 80% - non-condensing Atmospheric pressure 700 to 1060 hPa (Rated to be stored at an altitude ≤... -
Page 10: Cleaning & Infection Control
Part Number: SD01006 Iss 01 11/2023 Medi-Plinth plinths and couches must be stored in a dry environment, protected against ground moisture, away from direct sun or UV exposure and other direct sources of heat. If held in storage for long periods, a full service should be completed before plinth/couch is put back into service. -
Page 11: Decontamination
19. MAINTENANCE It is recommended that the Medi-Plinth products are serviced once per year. Particularly if in constant use. Regular service and maintenance are essential to maintain both the safety and reliability of the plinth/couch. All service and maintenance of the Medi-Plinth products must be completed by an authorised, competent and Medi-Plinth approved engineer who has been trained and certified by Medi-Plinth Equipment Ltd. -
Page 12: Troubleshooting
Regular servicing should be carried out a minimum of every 12 months or more frequently if the couch is in constant use. Routine servicing or repair must be carried out by an authorised, competent and Medi-Plinth approved engineer who has been trained and certified by Medi-Plinth Equipment Ltd. -
Page 13: Decommissioning And Disposal
Part Number: SD01006 Iss 01 11/2023 Check the gas strut still operates correctly. • Tighten locking nut to lock gas strut in place. 21. DECOMMISSIONING AND DISPOSAL When decommissioning either the plinth/couch or any accessory, please ensure the items are cleaned and disinfected, as described in the cleaning section before any other decommissioning activities. -
Page 14: Spare Parts
All spares can be purchased by the user from Medi-Plinth Equipment directly. Please contact the Medi-Plinth Equipment Limited sales team (sales@medi-plinth.co.uk) for further information. - Page 15 Do not use if the package is damaged and consult instructions for use Date of manufacture/Made in United Kingdom Authorized representative in the European Community/European Union Caution Catalogue number Serial Number Medical Device Temperature Limit Medi-Plinth serial number label. Medi-Plinth IFU Download label. Pg. 15...
- Page 16 Reproduction, adaptation, or translation without prior written permission is prohibited, except as allowed under the copyright laws. Thank you for choosing a Medi-Plinth Equipment Limited plinth/couch. Should you require any further details on the information contained in these instructions please contact us at: Company Registration No: 06204253 Medi-Plinth is an ISO 13485:2016 accredited company.




Need help?
Do you have a question about the Physio+ 2 Section and is the answer not in the manual?
Questions and answers