Subscribe to Our Youtube Channel
Summary of Contents for Richard Wolf PiezoWave2
- Page 1 Instructions for use PiezoWave2 Control Unit with PiezoWave2 Device Cart 100506/100905 GA-A342 / en / EU / V3.0 / 2021-09 / PK21-0170...
- Page 2 Richard Wolf reserves all rights in this document, in particular relating to reproduction, distribution, public communication and public dissemination. This document may not be modified, and texts and images must not be reproduced or translated without the express prior permission of Richard Wolf GmbH.
-
Page 3: Table Of Contents
Requirements for the products / components of a combination ..............15 Electromagnetic compatibility (EMC) - IEC 60601-1-2 : 2014 ..............17 Equipotentality ............................20 Illustration ..............................21 Figure: Front view of PiezoWave2 control unit ................... 21 Figure: Rear view of PiezoWave2 control unit ................... 22 Other figures............................... 23 8.3.1 Side view of the PiezoWave2 control unit .................... - Page 4 11.2.7 Resetting the treatment settings......................... 36 11.3 Operating the product..........................36 11.3.1 Treatment sequence ..........................36 11.3.2 Extended functions ............................. 37 11.3.3 Setting the extended parameter view ......................38 11.3.4 Setting T-burst frequency mode ......................... 38 11.3.5 Setting T-ramp frequency mode ......................... 39 11.3.6 Setting the control unit volume ........................
-
Page 5: General Information
General information Safety instructions and levels of danger WARNING This signal word is used to indicate a potentially dangerous situation. Not avoiding this situation can result in death or extremely serious injuries. CAUTION This signal word is used to indicate a potentially dangerous situation. Not avoiding this situation can result in minor or moderate injury. -
Page 6: Symbols
Symbols Symbols Designation Follow the instruction manual Medical device Off/On (supply, disconnection from/connection to the power supply) Equipotentality TYPE B APPLIED PART Fuse Alternating current (AC) Number of shockwaves Shockwave intensity Pulse rate T-burst frequency mode Pulse duration Pause duration T-ramp frequency mode Initial frequency Time frame... - Page 7 Symbols Designation Stop at Delete last input “Reset” button Substages activated/deactivated Volume loud/quiet Menu Confirm input Cancel input Therapy source Positive peak pressure Energy flux density Treatment duration Remaining treatment duration Check box not selected/selected Option field not selected/selected Secure area Footswitch Footswitch shockwave triggering Permissible total weight...
- Page 8 The CE identification on the title page of these instructions for use relates exclusively to the Richard Wolf main product or, if serveal identical products are described, to the Richard Wolf product with the highest classification. The CE identification of the other Richard Wolf products and, if applicable, of products made by other manufacturers, described in these instructions for use results exclusively from the identification on the product and/or packaging.
-
Page 9: General Safety Notes And Instructions For Use
Product description The PiezoWave2 is a compact extracorporeal shockwave therapy device that en- ables treatment with piezoelectric shockwaves. The PiezoWave2 features a sim- ple and intuitive user interface which allows the key treatment parameters to be set quickly and easily. -
Page 10: Intended Purpose
Intended purpose Control units for extracorporeal shockwave therapy (ESWT) sources 100506 PiezoWave2 Control Unit Active and reusable control units for extracorporeal shockwave therapy (ESWT) sources are used for short (under 60 minutes), non-invasive interventions. The products are used for controlling compatible piezoelectric therapy sources. -
Page 11: Indications
Indications The product is used in the disciplines of orthopedics, dermatology, and urology for extracorporeal shockwave therapy (ESWT) and trigger point shockwave ther- apy (TPST). Patient group For urology applications, use is restricted to male patients aged 14 or older. There is no restriction regarding ethnicity, height, weight, or other general condi- tions. -
Page 12: Contraindications And Side Effects
Contraindications and side effects Contraindications When using the products, the following general contraindications must be taken into account: Infections in focus Lungs in focus Environment of a pacemaker Arterial hypertension Clotting disorders (the patient’s coagulation status is required) or treatment with blood-thinning drugs Brain or nerves in focus (central nervous system) The following contraindications must be taken into account for use in urology:... -
Page 13: Side Effects
Side effects The following side effects must be taken into account for use in urology: Slight pain and petechiae. There is a possibility of short-term hematospermia (bloody semen), hematuria (reddish tinted urine), acute bacterial prostatitis, urinary tract infection, interim uri- nary retention (due to possible swelling of the prostate), or a temporary increase in PSA (prostate-specific antigen). -
Page 14: Combinations
(such as signal input connectors and signal output connectors for video signals, data exchange, controls) and the patient at the same time. The PiezoWave2 must only be used in conjunction with the therapy sources ap- proved by Richard Wolf and in accordance with the instruction manual of the ther- apy source used. -
Page 15: Requirements For The Products / Components Of A Combination
Requirements for the products / components of a combination The general requirements depend on whether the products/components are inside or outside the pa- tient environment. Medically used room Non-medically Requirements / measures used room within the patient envi- outside the patient Leakage currents to IEC/EN 60601-1 ronment environment... - Page 16 When connected via the same multiple socket strip under standard conditions, the earth leakage current of the socket strip must not exceed 5 mA. e.g. Richard Wolf video cart with "isolating transformer" Only connect devices with a safety extra-low voltage of no more than 60 V DC / 42.4 V AC peak to the connectors for electrical connections, i.e.
-
Page 17: Electromagnetic Compatibility (Emc) - Iec 60601-1-2 : 2014
Electromagnetic compatibility (EMC) - IEC 60601-1-2 : 2014 NOTE The device/system in the following called product always refers to the product mentioned in the chapter on "intended purpose". Guidelines and manufacturer's declaration - Electromagnetic emissions The product is intended for use in the environment specified below. The user must assure that the product is used in such an environment. - Page 18 Guidelines and manufacturer's declaration - Electromagnetic immunity The product is intended for use in the environment specified below. The user must assure that the product is used in such an environment. Immunity tests IEC 60601 test level Compliance Electromagnetic environment - Guidelines Electrostatic discharge (ESD) ±...
- Page 19 Guidelines and manufacturer's declaration - Electromagnetic immunity for products that are not life-supporting The device is intended for use in the electromagnetic environment defined below. The user must assure that the product is used in such an environment. Immunity tests Test level to IEC Compliance level Electromagnetic environment - Guidelines...
-
Page 20: Equipotentality
Recommended safety distances between portable and mobile HF telecommunication devices and the producte Definition relative to high-frequency wireless communication devices Frequency band (MHz) Test frequency (MHz) Modulation Compliance level (V/m) 380–390 Pulse – 18 Hz 430–470 FM ± 5 kHz deviation or pulse –... -
Page 21: Illustration
Illustration Figure: Front view of PiezoWave2 control unit Fig. 1 Connection socket for therapy source (type B Operating and display unit applied part) Holder for therapy source Tablet holder GA-A342... - Page 22 Figure: Rear view of PiezoWave2 control unit 1.14 1.16 1.15 1.11 1.12 1.13 1.17 Fig. 2 1.11 Power input connector with fuse holder 1.15 USB data interface (for service only) 1.12 Socket for footswitch 1.16 Fan grid with filter unit 1.13 Potential equalization connector 1.17...
-
Page 23: Other Figures
Other figures 8.3.1 Side view of the PiezoWave2 control unit 1.21 Fig. 3 1.21 Power switch GA-A342... -
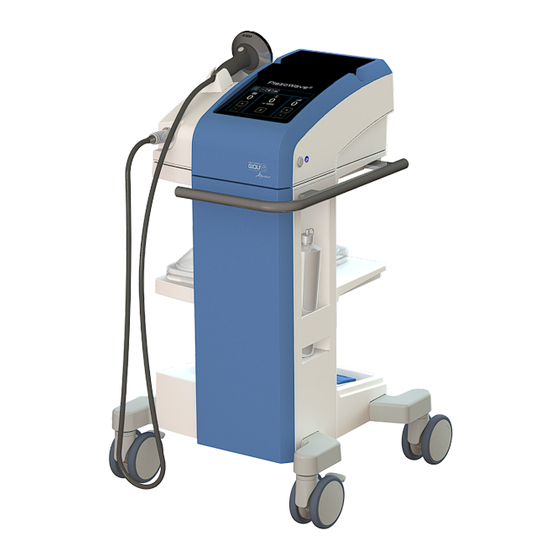
Page 24: View Of Piezowave2 Device Cart
8.3.2 View of PiezoWave2 device cart Fig. 4 Device cart Lower tray Device cart identification plate Double casters, front, with parking brake Stowage area for control unit Double casters, rear, with parking brake Shelf with fixation for gel pad trays GA-A342... -
Page 25: View Of Footswitch With 3 Pedals
8.3.3 View of footswitch with 3 pedals Fig. 5 Item Designation Item Designation Footswitch with 3 pedals Increase shockwave intensity “+” Shockwave triggering Identification plate Decrease shockwave intensity “-” GA-A342... -
Page 26: Checks
Checks CAUTION Injury due to damaged or incomplete products! Injuries of the patient, user and others are possible. Run through the checks before and after each use. Do not use the products if they are damaged or incomplete or have loose parts. -
Page 27: Preparation / Commissioning
Preparation / Commissioning WARNING Danger of explosion The device is not protected against explosions. Do not operate this device in areas where there is a danger of explosion. WARNING Risk of electric shock. Only connect the device to supply voltages that match the details on the identification plate. - Page 28 CAUTION Fire hazard or product damage. Do not cover or block ventilation slots required for cooling. ATTENTION Incorrect operation. Do not operate the devices side by side or stack them with other devices. If it is necessary to stack the devices or place them next to each other and HF interference is observed, make sure that the devices are operated as in- tended.
-
Page 29: Connection
10.1 Connection 10.1.1 Connecting the footswitch with 3 pedals ¯ Connect the footswitch plug to the socket on the rear panel of the control unit and screw home. 10.1.2 Connecting the therapy source NOTE To prepare the therapy source, observe the instruction manual of the therapy source used. -
Page 30: Preparation
10.2 Preparation CAUTION Danger of injury from moving device cart. Lock the parking brake before treatment. The parking brake must remain locked during treatment. Lock the parking brakes before and keep them locked when installing/remov- ing the control unit. 10.2.1 Device cart parking brakes The parking brakes on the double casters secure the device cart against rolling. -
Page 31: Installing The Control Unit On The Device Cart
10.2.3 Installing the control unit on the device cart 1. Carefully lower the control unit onto the top shelf of the device cart. ð Make sure that the feet of the control unit fully engage in the recesses of the top shelf. 2. -
Page 32: Use
11.1 General notes and instructions for use CAUTION Tissue/vascular damage. Only use the products if you have a clear view of the treatment area. CAUTION Danger of tipping over when moving the cart on inclined surfaces Observe the following: Do not exceed the maximum permissible load rating of the shelves. The therapy source must be secured in the therapy source holder. -
Page 33: Operating Principle
The system allows single pulse or continuous pulse mode. The intensity of the shockwave can be adjusted according to the patient in question. Fig. 11 11.2 Control elements and operating modes 11.2.1 Power switch The PiezoWave2 control unit is de-energized by switching off the power switch (disconnecting the power supply). GA-A342... -
Page 34: Connecting The Therapy Source
11.2.2 Connecting the therapy source ¯ The therapy source is not connected. ð Please connect a therapy source. Fig. 12 11.2.3 Menu structure Main menu Shockwave intensity Pulse rate Shockwave target sum T-ramp setting T-burst setting Extended view Main menu setting Speaker Volume adjustment Statistics... -
Page 35: Setting The Shockwave Intensity
11.2.4 Setting the shockwave intensity ¯ Use “+” and “-” to set the required shockwave intensity. Fig. 14 11.2.5 Setting the pulse rate ¯ Use “+” and “-” to set the required pulse rate. Fig. 15 11.2.6 Setting the shockwave target sum 1. -
Page 36: Resetting The Treatment Settings
11.2.7 Resetting the treatment settings ¯ This control button resets the current treatment settings. Fig. 17 11.3 Operating the product 11.3.1 Treatment sequence CAUTION Tissue/vascular damage. Observe the instruction manuals of the products used in combination with this product. 11.3.1.1 Shockwave therapy CAUTION Tissue and vascular damage. -
Page 37: Extended Functions
11.3.2 Extended functions 11.3.2.1 Setting the substages – intensity levels 0.0 to 0.9 Fig. 19 ¯ The substage control button is used to activate and deactivate the selection range of the lower intensity levels 0.0 to 0.9 GA-A342... -
Page 38: Setting The Extended Parameter View
11.3.3 Setting the extended parameter view NOTE Acoustic KPIs shown on the device display are measured under laboratory con- ditions according to IEC 61846 and may deviate from the displayed values under real conditions. Always pay attention to the patient’s feedback during treatment. Fig. 20 1. -
Page 39: Setting T-Ramp Frequency Mode
11.3.5 Setting T-ramp frequency mode In T-ramp frequency mode, shockwaves are emitted with an increase in fre- quency. When the shockwaves are activated, pulses are triggered at the initial frequency. The ramp function is activated by pressing the button for shockwave triggering again. -
Page 40: Taking Out Of Service
11.3.10 Taking out of service ¯ To take the device out of service, switch off the power switch and disconnect the device from the power supply / mains. ð To fully disconnect the device from the power supply / mains, it is neces- sary to disconnect the power cable. -
Page 41: Reprocessing And Maintenance
NOTE For chemicals whose material compatibility has been approved by Richard Wolf for the reprocessing of Richard Wolf products, please refer to the listing in docu- ment GA-J055. This document can be requested from Richard Wolf or down- loaded from our website. -
Page 42: Maintenance
Examples of suitable and approved surface disinfectants: Manufacturer Trade name Ingredient ® BODE Chemie, Hamburg Bacillol Propanol/ethanol ® B. Braun Melsungen Melliseptol sensitive Didecyldimethylammonium chloride, non-ionic surfactants Ecolab, Düsseldorf Incidin Alcohol Wipes 2-propanol & 1-propanol ® Schülke, Germany antifect N liquid Propanol/ethanol ®... -
Page 43: License Conditions
License conditions This product contains open source software of third party providers that are subject to the license agreements for the corresponding software packages. A storage medium with the software license agreements valid at the time of delivery and other contents, if applicable, is packed with the product. Please read these texts in detail for comprehensive information about your rights with regard to the aforementioned licenses. -
Page 44: Technical Description
Technical description 14.1 Troubleshooting ATTENTION If you cannot eliminate the faults with the help of this table, please call the ser- vice department or send in the device for repair. Do not attempt to do any repairs yourself. 14.1.1 Status and error messages on the display Message text Possible cause Corrective action... -
Page 45: Technical Data
Error Possible cause Corrective action No shockwave Footswitch is not connected or defec- Connect or replace the footswitch tive 14.2 Technical data 14.2.1 Technical data – control unit ESWT control unit Voltage Fre- Power consumption Current rating Fuse quency 100506 100–240 50/60 0.8–0.4... -
Page 46: Technical Data - Device Cart
The products can be combined as required provided the relevant technical data and intended uses are observed. For the general overview please refer to the latest catalog sheets and brochures, or contact Richard Wolf GmbH or your Richard Wolf representative. -
Page 47: Operating, Storage, Transport And Shipping Conditions
14.4 Operating, storage, transport and shipping conditions CAUTION Danger of injury due to product falling. Before transporting the device, secure all components against falling. ATTENTION Product damage. Use original packaging to transport the products. ATTENTION Product damage. Before transporting the device outside a building, disconnect the therapy source from the control unit and transport all components individually. -
Page 48: Replacing Parts
15.2 Replacing parts CAUTION Fire hazard or device malfunction. Only use the fuses specified in the spare parts list. Only use fuse ratings that correspond to those specified on the identification plate. Power input connector with fuse holder 1. Switch off the device and disconnect the power cable from the wall socket and from the power input connector of the device.

Need help?
Do you have a question about the PiezoWave2 and is the answer not in the manual?
Questions and answers