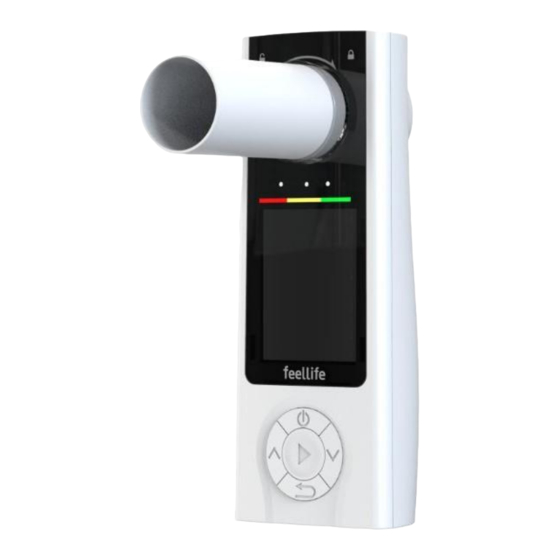
Summary of Contents for Feellife Air Smart T1
- Page 1 User Manual for SPIROMETER Model:Air Smart T1 Doc:LFS-EN-UM-24D Issue date:Apr.2,2021 Rev.:V1.0...
-
Page 2: Table Of Contents
Catalog 1. Intended Use ........................4 2. Working Principle ......................... 4 3. Product Contents and Overview ..................5 4. Technical Specification ......................5 5. Installation Guideline ......................6 6. Operating Instructions ......................8 7. Cleaning and Disinfection ....................18 8. Storage and Maintenance ....................19 9. - Page 3 ◆ Thank you for purchasing this product of FEELLIFE HEALTH INC. ◆ To ensure the correct use of this product, please read this instruction for use carefully before using. ◆ Please keep this instruction for use in a place where convenient for reading at any time.
-
Page 4: Intended Use
Safety Warning ◆ The use of this device for children and special groups like disabled,elderly must be carried out under the correct guidance and supervision. ◆ The user can only be used for the specified purpose, not for other purposes. ◆... -
Page 5: Product Contents And Overview
It is mainly composed of the Main Unit, Turbine,Disposable Mouthpiece and USB Cable. 3.2 Product composition 4. Technical Specification Product name Spirometer Model Air Smart T1 Internal power supply DC 3.7 V, 2000mAh, rechargeable lithium battery External power supply With DC 5 V, 1 A adapter Display mode... -
Page 6: Installation Guideline
Transportation and storage environment Temperature:-20℃~+55℃ Relative humidity:10%~93% R.H. (Non-condensing) Relative atmospheric pressure:70kPa~106kPa Operating environment Temperature:+5℃~+40℃ Relative humidity:15%~80% R.H. (Non-condensing) Relative atmospheric pressure:86kPa~106kPa Dimension(mm) 158(L)×60(W)×36.3(H) (Main Unit) Weight(g) 178.8 Applied part classified The classified of applied part is BF. Battery service life Battery service life can exceed 300 cycles. - Page 7 Caution: 1) The disposable mouthpiece is a paper material, it is designed for single user and single-use.It is not allowed for multi-users.
- Page 8 2) Any disposable mouthpieces included with the spirometer are only to be used as a reference guide to purchase the correct size mouthpiece required. These mouthpieces are clean but not sterile. To purchase appropriate mouthpieces, generally either paper or plastic.We suggest that you contact your local distributor. Warning: 1) Use a bio-compatible mouthpiece to avoid any problems to the patient.Unsuitable materials could cause the device to malfunction, consequently providing incorrect test...
-
Page 9: Operating Instructions
The battery symbol will flashes at the low-battery state. Please charge in time,otherwise this device will shuts down automatically. The fully charged lithium battery can work continuously for about 72 h. When it is not used for more than one month, it shall be charged at least once a month during the storage period. - Page 10 Measurement diagram • Activities that should be avoid before Lung Function Testing Smoking and/or vaping and/or water pipe use within 1 h before testing (to avoid acute bronchoconstriction due to smoke inhalation) Consuming intoxicants within 8 h before testing (to avoid problems in coordination,comprehension,and physical ability) Performing vigorous exercise within 1 h before testing (to avoid potential exercise- induced bronchoconstriction)
- Page 11 Figure 2 Testing interface Health status indicator bar Figure 3 Main parameter interface 3) Main parameter interface: display 8 parameter values and the ratio of each parameter to its corresponding predicted value. The ratio reflects health status, correct settings of personal information is the key to obtain accurate ratio. Besides, this interface also displays power icon, current time, case number and health status indicator, as shown in Figure 3.
- Page 12 Figure 4 Flow rate-volume chart and Volume-time chart 6.3 Menu In Testing interface,press CONFIRM key to enter Menu interface shown as Figure 5, "Personal Information", "Data Management", "Settings" and "Power Off" can be selected, press UP or DOWN key to select corresponding item, then press CONFIRM key to enter its sub-menu, methods are as followings: Figure 5 Menu interface ...
- Page 13 "Number"is the current case number. For example, if you are the 23 user, the "Number"will be 23. Case number can increase automatically, no need to set manually. 2) Setting Select "Gender" and press CONFIRM key.Press UP or DOWN key to select "MALE" or "FEMALE", then press CONFIRM key to confirm.
- Page 14 Health status indicator bar Figure 8 Review function interface 2) Trend Curve Select "Trend Curve" in Data Management interface as shown in Figure 9.After selecting the parameter, press CONFIRM key to enter Trend curve display interface, as shown in Figure 10.The figure is a summary of all stored data aiming at the selected parameter.It displays the trend change vividly, which is convenient for user to compare.
- Page 15 Figure 11 Delete selection interface 4) Denote Value Select "Denote Value" in Data Management interface as shown in Figure 12, after selecting the parameter, it will automatically return to Data Management interface.The Health status indicator will show in accordance with the parameter you set.(as shown to Figure 3) Figure 12 Denote value setting interface 5) Exit...
- Page 16 Figure 13 Settings interface 1) Language Select "Language" in Settings interface, then press UP or DOWN key to select "English" or "Chinese" (If this device does not have built-in language selection function, the operation is invalid). 2) Bluetooth Select "Language" in Settings interface, then press UP or DOWN key to select "ON"/"OFF".Collect your phone and this device via Bluetooth.You can review the data by this way.
- Page 17 3) Time setting Select "Time" to enter its setting interface, select "Year" to display current year as shown in Figure 14, press UP or DOWN key to change the value and press CONFIRM key to save. The operation steps of "Month", "Day", "Hour", "Minute" and "Second" is the same with the "Year".
- Page 18 In Menu interface, select "Exit" or press RETURN to return to Testing interface. 6.4 Charging The device will automatically enter the charging interface when it is charging. Under this interface,this device cannot be used. The indicator light on the top left of the device is displayed in orange when the device is charging,and it turns to green after the device is fully charged.
- Page 19 FEF75 Forced expired flow at 75% of FVC FEF2575 Forced expiratory flow between 25% and 75% of...
-
Page 20: Cleaning And Disinfection
7. Cleaning and Disinfection 7.1 Cleaning After each use, it is necessary to clean the turbine.The specific cleaning method is recommended as follows: • Remove the reusable turbine from the main unit by rotating it counter-clockwise and apply slight pressure with a finger from the bottom of the turbine to lift it out of its housing. -
Page 21: Storage And Maintenance
8) The turbine is forbidden to be washed under running water or be placed under direct air pressure.It is forbidden to be washed by hot fluids. 8. Storage and Maintenance 8.1 Storage conditions 1) Storage environment: please see “Transportation and storage environment” section for details. - Page 22 Acute cor pulmonale Clinically unstable pulmonary embolism History of syncope related to forced expiration/cough Due to increases in intracranial/intraocular pressure Cerebral aneurysm Brain surgery within 4 wk Recent concussion with continuing symptoms Eye surgery within 1 wk Due to increases in sinus and middle ear pressures Sinus surgery or middle ear surgery or infection within 1 wk Due to increases in intrathoracic and intraabdominal pressure Presence of pneumothorax...
-
Page 23: Trouble Shooting
6) Avoid placing in the environment that is high temperature, humidity and direct sunlight. 7) Please do not use volatile oil or other organic solvents for cleaning,so as to avoid damaging this device. 8) Do not drop or impact this device strongly,so as to avoid damaging it. 9) It is forbidden to dry this device in microwave oven. -
Page 24: Note & Warning
The device can not be The battery is damaged. Please contact the fully charged after manufacturer or distributor. charging more than 10 hours. 11. Note & Warning 11.1 Note and suggestion Spirometer is a medical device,please read the user manual before using it. •... - Page 25 The USB interface is only used for charging and cannot be connected to other • devices. The patient is the intended operator and all functions of this device can be used by • patients. The maintenance the user can perform are cleaning and disinfection. You can refer •...
-
Page 26: After-Sales Service
Replacement of lithium batteries or fuel cells when incorrect replacement would • result in an unacceptable RISK. The device needs to be kept out of the reach of children or pets, to prevent animal • hair or dirt entering the turbine to affect its use. 12. -
Page 27: Symbol Description & Safety Regulations & Electromagnetic Compatibility
of charge.All consumable parts,including reusable turbine,disposable mouthpiece are excluded from the terms of this guarantee. 2) Users can contact after-sales service if they have any questions during use. 3) If necessary, the circuit diagram and necessary data for repair can be provided to assist service personnel. - Page 28 Medical device Charging indicator 13.2 Safety requirements 1) Classification by type of anti shock: internal power supply. Classification according to the degree of anti electric shock: type BF application part. Degree of protection provided by enclosure: IP22 2) According to the disturbance characteristics of Industrial Science and medical (ISM) radio frequency equipment, it is classified into 1 group and B group.
- Page 29 or the user of this device should assure that it is used in such an environment. Electromagnetic environment – Emissions test Compliance guidance This device use RF energy only for its internal function. Therefore, its RF emissions RF emissions CISPR 11 Group 1 are very low and are not likely to cause any interference in...
- Page 30 . ) for 1 cycle . ) for 1 cycle recommended that the device be powered 70 % U 70 % U from an (30% dip in U ) for (30% dip in U ) for uninterruptible 25/30 cycles 25/30 cycles power supply or <5 % U (>95 % dip in...
- Page 31 recommended separation distance in meters(m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,*2) should be less than the compliance level in each frequency range.*3) Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
-
Page 32: Configuration List
0.38 0.38 0.73 0.12 0.12 0.23 0.01 For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. -
Page 33: Manufacturer And Rep. Information
(with the store and address). Any repair service out of the scope of warranty will be charged accordingly. 16. Manufacturer and Rep. information FEELLIFE HEALTH INC. Room 1903, Building A, No.9 Furong Road, Tantou Community, Songgang Subdistrict, Bao’an District, Shenzhen, 518104 Guangdong, P.R. China Tel:+86-755-68867080 E-mail:info@feellife.com... - Page 34 FCC Warning This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation. Any Changes or modifications not expressly approved by the party responsible for compliance could void the user's authority to operate the equipment.

Need help?
Do you have a question about the Air Smart T1 and is the answer not in the manual?
Questions and answers