Summary of Contents for I-MED I-PEN
- Page 1 I-PEN ® Tear Osmolarity System FOR QUANTITATIVE MEASUREMENT OF OSMOLARITY OF OCULAR TISSUES USER MANUAL www.imedpharma.com...
- Page 2 ® TEAR OSMOLARITY SYSTEM CONTACT INFORMATION To help us in providing you with the best possible product and support, please send your comments or questions to info@imedpharma.com I-MED Pharma Inc. 7190 Frederick Banting Rd. St. Laurent, QC Canada H4S 2A1...
-
Page 3: Table Of Contents
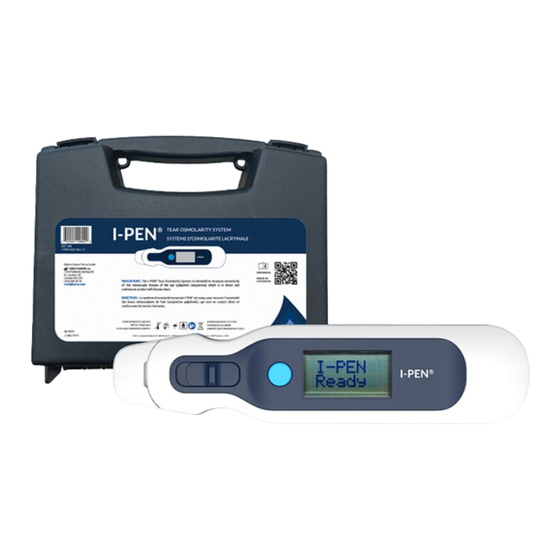
Device ® 3.3. I-PEN Single-Use-Sensor ® 4. PERFORMING A TEAR OSMOLARITY MEASUREMENT STEP 1. Prepare the I-PEN Tear Osmolarity System for Use ® 1.1. Insert the Battery STEP 2. Remove the Single-Use-Sensor from Package STEP 3. Insert the Single-Use-Sensor STEP 4. Taking a Reading STEP 5. -
Page 4: About This Manual
Tear Osmolarity System User Guide ABOUT THIS MANUAL ® 1. ABOUT THIS MANUAL This manual provides the information necessary to operate the I-PEN Tear Osmolarity System in ® a safe and efficient manner. Please read and thoroughly understand this manual before operating the system. -
Page 5: Essential Prescribing Information
After several seconds of firm contact with the eyelid tissue, the I-PEN Tear Osmolarity System will ® display a quantitative tear osmolarity test result on the liquid crystal display (LCD). The I-PEN Tear ® Osmolarity System simplifies the osmolarity determination process by eliminating the need to transfer tear fluid samples and reducing the risk of evaporation, reading errors due to temperature fluctuations and the need for calibration. -
Page 6: General Safety Instructions
® 2.5. GENERAL SAFETY INSTRUCTIONS WARNING: Changes or modifications not expressly approved by I-MED Pharma Inc. can affect the safety and effectiveness of the system and will void the system’s warranty. WARNING: Use only indoors, in a clean, dry environment. -
Page 7: Description Of Components
Tear Osmolarity System indicates a Low Battery message, it is required to wait ® for a minimum of two minutes without using the device. After the waiting period, try using the I-PEN ® Tear Osmolarity System again. If the Low Battery message persists, it is advised to replace the battery. -
Page 8: Performing A Tear Osmolarity Measurement
Tear Osmolarity System indicates a Low Battery message, it is required to wait ® for a minimum of two minutes without using the device. After the waiting period, try using the I-PEN ® Tear Osmolarity System again. If the Low Battery message persists, it is advised to replace the battery. -
Page 9: Step 2. Remove The Single-Use-Sensor From Package
• Replace the device if a “beep” is not heard after turning it on. • It is important to visually inspect the I-PEN Single-Use-Sensor before use. In the case of suspected ® contamination, or if the expiration date is expired, replace the I-PEN Single-Use-Sensor. ® CAUTION: Insert the I-PEN Single-Use-Sensor without touching the gold nodes. -
Page 10: Step 4. Taking A Reading
“beep” and the LCD display should display the “I-PEN Ready” message. 4. Approach at a 30-45 degree angle from horizontal and gently lower the end of the I-PEN ® Single-Use-Sensor on to the middle of the conjunctiva on the inside of the lower eyelid. -
Page 11: Single-Use-Sensor
I-PEN Tear Osmolarity System User Guide RESULTS INTERPRETATION ® STEP 5. EJECT THE I-PEN SINGLE-USE-SENSOR ® Push the Ejector button and the I-PEN Single- ® Use-Sensor will be ejected. You may now discard the I-PEN Single-Use-Sensor. ® The LCD will display a test result. -
Page 12: Cleaning And Maintenance
Cleaning fluids should never be used on the I-PEN Single-Use-Sensor. ® 6.1.2. I-PEN SINGLE-USE-SENSORS ® I-PEN Single-Use-Sensors are for use on a single eye. Do not re-use or try to clean an I-PEN Single- ® ® Use-Sensor. 6.2. MAINTENANCE The I-PEN Tear Osmolarity System is designed to work without direct service or preventive maintenance. -
Page 13: Operating And Storage Conditions
Degree of Protection Against Electric Shock BF Type applied part W 34.5 mm Size (not including probe holder) L 142.5 mm H 18 mm Weight 50 gm CR2032 - The I-PEN battery complies with IEC ® Battery 60086-4 Frequency 80 Hz Peak Voltage ±... -
Page 14: Electromagnetic Emissions
Tear Osmolarity System User Guide ELECTROMAGNETIC EMISSIONS ® 9. ELECTROMAGNETIC EMISSIONS • The I-PEN Tear Osmolarity System requires special precautions with regard to electromagnetic ® compatibility. It must be installed and prepared for use as described in Section 4. Performing a Tear Osmolarity •... -
Page 15: Electromagnetic Immunity
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The I-PEN Tear Osmolarity System is intended for use in the electromagnetic environment specified ® below. The customer or the user of the I-PEN Tear Osmolarity System should assure that it is used in ® such an environment. - Page 16 Tear Osmolarity System is used exceeds the applicable RF compliance level above, ® the I-PEN Tear Osmolarity System should be observed to verify normal operation. If abnormal performance is observed, additional ® measures may be necessary, such as re-orienting or relocating the I-PEN Tear Osmolarity System. ®...
-
Page 17: Recommended Separation Distances
The user and/or installer of the unit can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile radio frequency communications equipment (emitters) and the I-PEN Tear Osmolarity System, according to the maximum output power of the ®... -
Page 18: Labels And Symbols
LABELS AND SYMBOLS ® 11. LABELS AND SYMBOLS 11.1. LABELS 11.2. SYMBOLS A number of internationally recognized symbols are found on the I-PEN unit. ® These relate to safety requirements and standards and are briefly reviewed below. Symbol Symbol meaning... - Page 19 I-PEN Tear Osmolarity System User Guide ® Manufactured by: 7190 Frederick Banting Rd. St. Laurent, QC Canada H4S 2A1 Tel: (514) 685-8118 Toll Free: (800) 463-1008 Email: info@imedpharma.com Website: www.imedpharma.com...
- Page 20 I-MED Pharma Inc. 7190 Frederick Banting Rd. St. Laurent, QC Canada H4S 2A1 Tel: (514) 685-8118 Toll Free: (800) 463-1008 info@imedpharma.com Product #IPUMEN 0424...


Need help?
Do you have a question about the I-PEN and is the answer not in the manual?
Questions and answers