Table of Contents

Summary of Contents for ROLENCE ENTERPRISE ELiTEDENT MS-30
- Page 1 User Manual ® ® ROLENCE ELiTEDENT ULTRASONIC SCALER MS-30 ROLENCE ENTERPRISE INC. No. 18-3, Lane 231, Pu Chung Rd., Chungli, Taoyuan 32083, Taiwan TEL: +886 3-4631999 FAX: +886 3-4631997 www.rolence.com.tw S-19-01-1218-U0001 V1.5...
-
Page 2: Table Of Contents
Table Of Contents Section Section Title Page Number Description of Contents Precautions……………………………………………………………………………………………..3 1.1 Precautions for All Systems 1.2 Precautions for Ultrasonic Prophylaxis Procedure Introduction…………………………………………………………………………………………..4 Glossary of Symbols……………………………………………………………………………..4 Intended Use……………………………………………………………………………………………5 Specification………………………………………….………………………………………………...5 Contraindications and Warnings…………………………………………………………………5 6.1 Contraindications 6.2 Warnings Infection Control……………………………………………………………………………………….6 7.1 Function 7.2 General Infection Control Recommendations 7.3 Water Supply Recommendations Installation Instructions……………………………………………………………………………7... -
Page 3: Precautions
Disclaimer……………………………………………………………………………………………...12 WARRANTY………………………………….………………………………………………………...13 Section 1: Safety Precautions Prior to installation and start-up of the ultrasonic scaler, carefully read the instructions provided herein! 1.1 Precautions for All Systems Do not place the ultrasonic scaler on or next to a radiator or other heat source. Excessive heat may damage the ultrasonic scaler’s electronics. -
Page 4: Introduction
Retract the lips, cheeks and tongue to prevent contact with the ultrasonic tip whenever it is placed in the patient's mouth. Section 2: Introduction Rolence Enterprise Inc. is an ISO 13485 certified 2.2 Supplies and Replacement Parts manufacturer of Ultrasonic Scalers. All the products... -
Page 5: Intended Use
The equipment complies with the requirements in the Medical Device 1434 Directive 93/42 EEC. Icon to identify electric and electronic devices. The unit must be collected and disposed of separately. Section 4: Intended Use Ultrasonic procedure A ll general supra and subgingival scaling applications. ‧... -
Page 6: Infection Control
electronic equipment might interfere with the operation of the device. We recommend that the During using the unit, make sure that water is ‧ MS handpiece cable kept at least 6 to 9 inches (15 flowing continuously. If the handpiece overheats to 23 cm) away from any device and their leads please check the water supply, and stop using the during use. -
Page 7: Installation Instructions
Section 8: Installation Instructions 8.1 General Information 8.3 Electrical Requirements Refer to Section 3: Specifications. ® If the installation of your ELiTEDENT MS-30 ultrasonic scaler is performed by someone other 8.4 Unpacking the System ® ® than trained Rolence distributor personnel, care Carefully unpack your ELiTEDENT ultrasonic should be taken to observe the following... -
Page 8: Elitedent
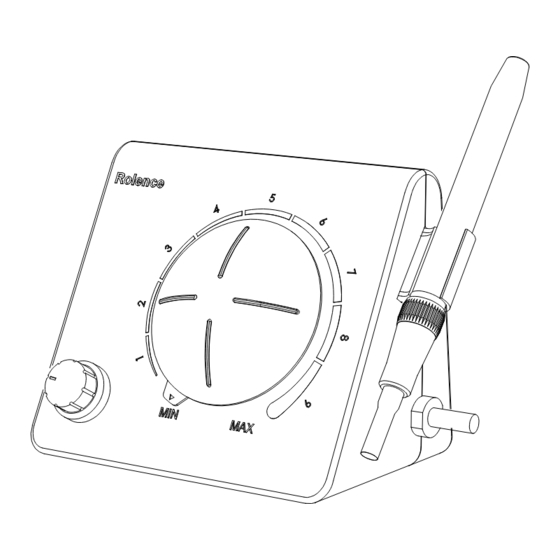
® Section 9: ELiTEDENT Ultrasonic Scaler Description 9.1 Rear Panel Power Switch Solenoid Water Inlet Foot Pedal Connector Power Inlet 9.2 System Control Turbo Indicator Light Sterilizable Sleeve Power Adjustment Lit when Foot Switch is fully of Handpiece Turn the knob to select power depressed, and System is Operates Ultrasonic level for operation: clockwise... -
Page 9: Accessories
9.3 MS Handpiece Cable Set / Sterilizable Sleeve of Handpiece Assembly ® The ultrasonic scaler is multi-frequency unit compatible with all Cavitron inserts. The system will automatically detect the insert, no need to switch any button. For more oral hygiene care, the handpiece sleeve can be dismantled and autoclaved. See below: Sterilizavle Sleeve of Handpiece Enable to dismantle... -
Page 10: Techniques For Use
Section 11: Techniques For Use 11.1 Patient Positioning It may be necessary to adjust water flow larger ‧ For optimal access to both the upper and lower under "Turbo" mode (Foot Pedal fully depressed) ‧ arches, the backrest of the chair should be so adequate fluid will be available to cool tip and adjusted to a 45 degree angle. -
Page 11: System Maintenance And Care
Section 12: System Maintenance and Care 3. Place a sterilized sterilizable sleeve of handpiece. Daily Maintenance It is recommended that you perform the following Set power to minimum. Hold the sterilizable maintenance procedures to help minimize bio-film sleeve of handpiece over a sink or drain and flush ®... -
Page 12: Trouble Shooting
are preferred, due some alcohol-based disinfectant shrinkage tube which protected by sterilizable solutions may be harmful and may discolor plastic sleeve of handpiece is very sensitive to the materials. disinfectant solution water. After disinfection, wipe surface of shrinkage tube with a *Note: The electric wire winding covered by heat slightly damp cloth and dry thoroughly before use. - Page 13 (This instruction subjects to change without pre-notice.) The equipment complies with the requirements in the Medical Device Directive 93/42 EEC. 1434 Rolence Enterprise Inc. No. 18-3, Lane 231, Pu Chung Rd., Chungli, Taoyuan 32083, Taiwan. EU authorized representative name and address CMC Medical Devices &...
- Page 14 ANNEX I Guidance and manufacturer’s declaration-electromagnetic emissions The MS-30 is intended for use in the electromagnetic environment specified below. The customer or the user of the MS-30 should assure that it is used in such an environment. Emission test Compliance Electromagnetic environment-guidance RF emissions Group 1...
- Page 15 supply 70% UT(30% dip in 70% UT(30% dip in operation during power mains input lines IEC UT) for 25 cycles UT) for 25 cycles interruptions, it is recommended 61000-4-11 <5% UT(>95% dip in <5% UT(>95% dip in that the MS-30 be powered UT) for 5 s UT) for 5 s from an uninterruptible power...
- Page 16 NOTE1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.

Need help?
Do you have a question about the ELiTEDENT MS-30 and is the answer not in the manual?
Questions and answers