Summary of Contents for STORZ MEDICAL MASTERPULS MP100
- Page 1 MASTERPULS MP100 ® 0S.#### Part No. 23232.0100 Published: July 2015 Original language: German Publisher: STORZ MEDICAL AG Lohstampfestr. 8 CH-8274 Tägerwilen Switzerland 24523...
-
Page 2: Table Of Contents
Table of Contents General Safety Information Instructions for safe use 1.1.1 Designated use and operational safety. 1.1.2 Safety during treatment of the patient. Warning against damage to equipment and the device Principles Physical principles 2.1.1 Indications . 2.1.2 Contraindications . 2.1.3 Side effects . - Page 3 4.4.4 Treatment menu bar . . 24 4.4.5 Device info and settings menu bar . . 25 Touch screen operation 4.5.1 Set and reset device type . . 26 4.5.2 Password protection . . 27 4.5.3 Setting brightness and volume . .
- Page 4 Cleaning, Maintenance, Overhaul Cleaning 5.1.5 Cleaning the tablet . 52 5.1.1 Cleaning of the handpieces . . 52 5.1.2 Fuse replacement . . 53 Maintenance and safety checks Disposal Repair Service life Accessories Technical Specifications 7.1 Technical Specifications Type plate MASTERPULS MP100 ®...
- Page 5 Preface Warning notes This manual contains warnings, safety instructions and specific operating instructions in accordance with liability regulations. DANGER refers to a situation of acute danger which, if not avoided, could lead to serious or fatal injury. DANGER! The source of the danger is stated here. These are the possible consequences! •...
-
Page 6: General Safety Information
The device is only allowed to be used for the applications described in C 2.1.1 hapter ndiCatiOns • Only perform treatments approved by STORZ MEDICAL AG! Furthermore, the device is only allowed to be operated by trained personnel who comply with the p in C 2.2. -
Page 7: Safety During Treatment Of The Patient
Shock waves can give rise to undesirable heart reactions. The patient must be continuously observed during the treatment. Only perform treatments approved by STORZ MEDICAL AG! The user is responsible for correctly positioning the handpieces and correctly selecting the treatment zone. - Page 8 The MASTERPULS ® MP100 is not allowed to be positioned immediately next to or jointly with other devices. If the operation near or jointly with other devices is required, the MASTERPULS MP100 must be tested in that particular environment to ®...
-
Page 9: Principles
Principles Physical principles The MASTERPULS MP100 is a compressed air–operated ballistic shock wave genera- ® tor. The shock waves in the MASTERPULS MP100 are generated with a precision ® ballistic mechanism in the handpiece. A projectile is accelerated by compressed air. The motion and weight of the projectile produce kinetic energy. -
Page 10: Side Effects
Treatment with the STORZ MEDICAL MASTERPULS ® MP100 is not permitted in the following cases: – Coagulation disorders (haemophilia) – Use of anticoagulants, especially Marcumar – Thrombosis – Tumour diseases, carcinoma patients – Pregnancy – Epiphyseal fusion areas in children –... -
Page 11: Training Of The Operator
An introduction to the principles of operation will be provided by your STORZ MEDICAL dealer with reference to this operating manual and will be documented in the system logbook. -
Page 12: System Description
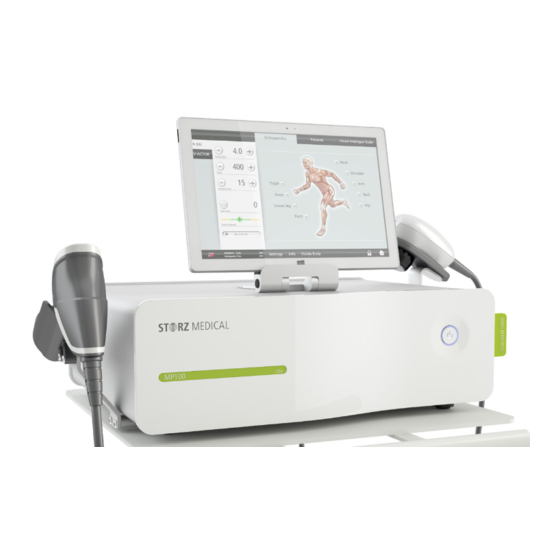
System Description Control and functional elements The MASTERPULS ® MP100 is controlled using the operating and display elements on the handpiece as well as using the SMAG tablet display. 1 main switch Fig. 3-1 Front side of MASTERPULS ® MP100 Fig. -
Page 13: Scope Of Supply
Scope of supply The standard scope of supply of the STORZ MEDICAL MASTERPULS ® MP100 includes the following items: – MASTERPULS MP100 control device ® – Mains cable (EU / USA) – Gel bottle – User manual (operating manual, system logbook and training records) –... -
Page 14: Installation Instructions
Installation Instructions 3.4.1 Mounting the handpiece holder There are two different handpiece holder: – for handpieces R-SW – for handpieces V-ACTOR. 1 Openings for insertion of the handpiece holder Fig. 3-3 Mounting the handpiece holder The mounting of the handpiece holder is equal for both models. •... -
Page 15: Connecting Handpiece
ATTENTION ! When setting up the instrument, make sure that the air outlets on the housing of the MASTERPULS MP100 are not blocked. ® The instrument must only be connected to properly earthed and correctly installed shockproof sockets! The device must be positioned in a way so that disconnection from the mains is easy to do. -
Page 16: Connecting Tablet
24040.02 or higher the software of the handpiece corresponds version 24264.03 or higher. Compatibility The STORZ MEDICAL Masterpuls MP100 is allowed to be operated with the following handpieces: – handpiece R-SW part.no. 21700_xxxx –... -
Page 17: Operation
When switching on the tablet the software starts but treatment is not possible. Make sure that both devices are switched on. Options for operation The following options for the use of MASTERPULS MP100 are available: – over the display of the handpiece – over the touch screen of the tablet... -
Page 18: Operation Of The Handpiece
Operation of the handpiece This device can be controlled directly using the handpiece. Corresponding setting buttons can be used for selecting the treatment parameters. The indicator window shows which setting has been selected. 1 decrease energy 2 increase energy 3 decrease frequency 4 increase frequency 5 Trigger button 6 Display energy... -
Page 19: Symbols And Displays
Symbols and displays The MP100 can be optionally controlled by the optional tablet when using the R-SW handpiece as well as the V-ACTOR handpiece. This allows also an individual adjust- ment of the parameters for the V-ACTOR handpiece. The user interface is divided into various areas: Menu bar: Monitor Monitor Counter... -
Page 20: Module Selection
4.4.1 Module selection The field at the top left is used for displaying the operating modes that can be select- ed. Once a handpiece is connected, it is possible to activate the corresponding operat- ing mode in the module selection area. The active module button is highlighted. Fig. -
Page 21: Parameter Selection And Counter Readings
4.4.2 Parameter selection and counter readings The PARAMETER SELECTION field is used for displaying and setting the treatment parameters. This is where you define the energy level as well as the number and fre- quency of the pulses before each treatment. Fig. -
Page 22: Contact Intensity And Skin Touch Display
4.4.3 Contact intensity and Skin Touch display You can track the contact intensity on a scale during the R-SW treatment. The indicator of the intensity on the scale will begin to move as soon as you touch the treatment zone with the R-SW shock transmitter. When the cursor is in the green range, the contact intensity for the treatment corresponds to the recommended intensity for the treatment. - Page 23 CAUTION! The shock transmitter surface will become hot! Extended skin contact can lead to minor burns! • Interrupt treatment after a maximum of 6,000 shocks. Do not apply more than 300-400 shocks to the same spot during treatment. • Place the handpiece back in the handpiece holder after the treatment. The Skin Touch option is switched off automatically after 10 minutes (with activation of the screen saver) if no treatment has taken place during this time.
- Page 24 4.4.4 Treatment menu bar Use the TREATMENT menu bar to call up stored treatment parameters and treat- ment reports as indications. Fig. 4-7 Treatment menu bar Buttons Meaning The ANATOMY view appears automatically when the Markers for various treatment zones device is started.
- Page 25 4.4.5 Device info and settings menu bar The bottom navigation bar contains control buttons used for navigating through the menus: – Software update – Options – Service VERSIONS – Serial number and indices of the individual components OPERATING DATA – Total pulse count and device operating hours (depending on operating mode selected) –...
- Page 26 Touch screen operation 4.5.1 Set and reset device type When starting the tablet the first time the software need to be adapted to the control device. When using the tablet with an other control device the device type needs to be reset- ted and adapted to this control device.
- Page 27 • Type password „RESET“ ( in capital letters) and confirm with Fig. 4-10 Reset system configuration • Confirm „Reset system configuration?“ by pressing • Now start the tablet anew. 4.5.2 Password protection It is possible to protect your SMAG tablet with a password. If starting the tablet anew or if it is in screen saver mode so the display is locked and can only be unlocked by entering the password.
- Page 28 Fig. 4-12 Password entry • Confirm with h Your password is activated. Deactivate password protection • Press several seconds on date and time field • Press to deactivate password entry (the check mark needs to be can- celled after that). •...
- Page 29 4.5.3 Setting brightness and volume • Select • Select in the top menu bar. Fig. 4-14 Options • Tap on the scale at the desired position in order to adjust the brightness of the screen or the volume. • Press to save the settings.
- Page 30 4.5.5 Selecting treatment parameters You can set the treatment parameters manually or load a predefined indication. For manual selection • Set the energy level as well as the number and frequency of the pulses using the buttons in the PARAMETER SELECTION field. h The treatment is now carried out with the displayed values.
- Page 31 Fig. 4-17 Selectable treatment zones – The alphabetically sorted list of indications for this treatment zone is opened. Navigating in the list Use the navigation bar on the right edge of the display to move within the list. 1 Navigation bar Fig.
- Page 32 Fig. 4-19 Overview - information about stored indications Calling up detailed views: For increased clarity, you can call up magnified views of the treatment photos as well as the recommended shock transmitters and stand-off devices. • Press the corresponding treatment photo. –...
- Page 33 Fig. 4-21 D20 detailed view Use the buttons to switch to the display of the previous or next element. • Press to switch to the treatment step overview. Loading treatment steps • Load the first treatment step using the button in front of it. –...
- Page 34 Loading a patient dataset You can now call up a patient record directly from the loaded indication. • Press – The list of stored patient data is opened. • Load the required dataset by pressing – The name of the patient is displayed in the status bar with the loaded indication.
- Page 35 • Use the onscreen keypad to enter an indication name and treatment region. • Save your entry by pressing h Your indication is now created in the system. If you press the button to return to the overview, you will see your new indication in the list. 4.5.8 Copying indications A copy of a preprogrammed indication can also be created.
- Page 36 4.5.9 Deleting an indication • Press – The list of indications is displayed. • Select the indication that you want to delete by pressing the button in front of it. – The indication is opened. DELETE INDICATION. • Press • Confirm your input by pressing NOTE This only applies to your own indications.
- Page 37 4.5.10.1 Storing treatment notes • To add remarks to the indication, press in the line. Using the onscreen keypad, you can now enter your remarks and notes in the text box. • Save your text by pressing h The text appears in the overview window of the indication. 4.5.10.2 Loading images and/or videos Not only images but also videos can be loaded in WMV format.
- Page 38 Deleting pictures and/or videos • To remove a picture or a video from the picture line, press on the symbol in the picture and confirm your entry with h The picture or video is deleted from the indication. 4.5.10.3 Creating, deleting or editing treatment steps •...
- Page 39 4.5.11 Patient treatment report Each treatment of a patient can be recorded in a treatment report and stored. 4.5.11.1 Loading patient data • Press in the top menu bar. – The alphabetically sorted list of patient data is opened. Fig. 4-30 List of stored patient data Navigating in the list Use the navigation bar on the right edge of the display to move within the list.
- Page 40 Fig. 4-31 Patient data • In the TREATMENTS line, press the button to call up details. – You can now see which parameters have been used for the patient’s treatment. Fig. 4-32 Treatment parameters used 19 300 02 0715...
- Page 41 4.5.11.2 Editing patient data You can add additional notes or treatment pictures by setting the dataset to editing mode. • Press the button to do this. The buttons with the pencil icon indicate the areas that can be edited. You can now: –...
- Page 42 4.5.12 Creating new patient data • Press in the top menu bar. – The alphabetically sorted list of patients is opened. • Press – A window with a keypad and text boxes for the patient data is opened. Fig. 4-34 Creating a new patient •...
- Page 43 4.5.13 Exporting treatment data This function can be used to export treatment data to a USB memory stick as Excel-readable files. • Ensure that your USB stick supports the USB V1.1 protocol. You can order a validated USB stick from your dealer. •...
- Page 44 4.5.16 Software updates • Before performing software update download the update. • Extract the files. • Load the extracted files onto an USB stick. • Press • In the open menu list, select the function. • Insert the USB stick into the slot (of the tablet and confirm with –...
- Page 45 symbol before an indication group indicates that it is activated. • If you want to switch to another indication group, press the in front of it. • Confirm by pressing the button. 4.5.18 Visible Body - Anatomy Atlas Visible Body is an interactive 3D atlas of the human body with which the musculature of the entire body and the individual muscle groups can be represented.
- Page 46 Fig. 4-38 Visible Body - Selection of the muscle region 4.5.18.2 Marking treatment regions • To load the marking pencil, press Fig. 4-39 Visible Body - Marking the treatment region • Now draw your markings of the region on the control panel. 19 300 02 0715...
- Page 47 Fig. 4-40 Visible Body - Marked region • Press the symbol to create a screenshot. It will be saved automatically under the currently opened patient data. 4.5.18.3 Exiting Visible Body • To exit the program, press on a module selection field (R-SW) or the empty area located below on the left that is outside the Visible Body screen.
- Page 48 Setting treatment parameters • Set the treatment parameters by pressing the buttons on the hand- spiece or the screen touch of the tablet. – Each selected nominal value is shown on the display. • Reset the shock wave counter as on the handpiece by pressing simultaneously button 1 and 3 on the standard dis- play (see Fig.
- Page 49 Functional checks Perform the following functional checks after the system has been installed: • Check the device and the handpieces for any signs of damage. • Put the device into operation. • Set the energy level to 2 bar. • Reset the treatment shock counter on the handpiece display. •...
- Page 50 4.10 Treatment CAUTION! The transport bag is provided only to transport the device. If the device is left in the transport bag during treatment, the device beco- mes hot, due to lack of ventilation. Burns, conflagration and damages of the device are possible. • Take the device out of the transport bag during treatment. Safety information Before using the device, the user must make sure it is functioning safely and in proper condition.
- Page 51 4.10.1 Setting parameters Treatment should always start at a low energy level. This also applies to resuming treatment after an interruption. The shock wave energy should be increased gradually during treatment. The low levels are used less for therapy and more for familiarising the patient.
- Page 52 Cleaning, Maintenance, Overhaul Cleaning Regular cleaning of the system ensures perfect hygiene and operation of the Masterpuls ® MP100. CAUTION! Electrical hazard! Disconnect the device and the accessories from the mains before starting any cleaning and overhauling work! Overall external cleaning depends on the frequency of use and application of the device.
- Page 53 Maintenance services can be ordered from our regional representatives in your area or directly from STORZ MEDICAL AG. Independently of the national accident prevention regulations and test and inspection intervals prescribed for medical devices, we recommend that functional checks (see 4.8 f...
- Page 54 Repair work on defective devices must only be carried out by personnel suitably authorised by STORZ MEDICAL. Only original STORZ MEDICAL spare parts may be used for this purpose. The personnel suitably authorised can be from STORZ MEDICAL or be representatives of STORZ MEDICAL agencies and dealers.
- Page 55 Accessories Mains cable CEE 4 m long 13455 Mains cable CH 3 m long 13448 R-SW handpiece set 21700.0001 R-SW overhaul kit 17212 A6 shock transmitter 17675 T10 shock transmitter set 13457 R15 shock transmitter 17638 C15 shock transmitter 19222 F15 shock transmitter 21356 DI15 shock transmitter...
- Page 56 Technical Specifications Technical Specifications MASTERPULS MP100 ® single shock, R-SW operating mode continuous shock 1-21 Hz/1-5 bar in steps of 0,1 bar without tablet - fixed: 31 Hz / 2,4 bar V-ACTOR operating mode with tablet - adjustable: 1-31 HZ / 1-5 bar Mains input voltage 100 - 240 VAC Mains frequency...
- Page 57 NOTE When the medical product is distributed to third parties, the following must be observed: – The complete device documentation must be delivered together with the medical product. – The medical product may only be exported to a foreign country when the medical product and the corresponding indications are allowed there.
- Page 58 7.4.1 EMC guidelines and manufacturer’s declaration Guidelines and manufacturer's declaration – emitted electromagnetic interference The MASTERPULS MP100 model is intended for operation in the electromagnetic environment specified ® below. The customer or the user of the MASTERPULS ® MP100 should ensure that it is used in such an environment.
- Page 59 Guidelines and manufacturer's declaration – Resistance to emitted electromagnetic interference ® The MASTERPULS MP100 model is intended for operation in the electromagnetic environment specified ® below. The customer or the user of the MASTERPULS MP100 should ensure that it is used in such an environment.
- Page 60 Guidelines and manufacturer's declaration – Resistance to emitted electromagnetic interference ® The MASTERPULS MP100 model is intended for operation in the electromagnetic environment specified ® below. The customer or the user of the MASTERPULS MP100 should ensure that it is used in such an environment.
- Page 61 Recommended safety distances between portable and mobile HF communications equipment and the MASTERPULS MP100 ® ® The MASTERPULS MP100 is intended for use in an electromagnetic environment in which radiated HF ® disturbances are controlled. The customer or the user of the MASTERPULS MP100 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile HF ®...
- Page 62 Certificates Fig. 7-43 Declaration of conformity 19 300 02 0715...
- Page 63 Symbols and labels The following symbols and labels are attached to the Masterpuls ® MP100: Label Name Type plate You must read the operating manual CSA certification mark WEEE symbol Tabelle 1-6 Labelling 19 300 02 0715...
- Page 64 Transport costs and the risk of loss during the shipping of returned products shall be borne by the customer. Please complete the attached warranty card and return it as soon as possible to the address below: STORZ MEDICAL AG Lohstampfestrasse 8 CH-8274 Tägerwilen Warranty for the handpiece The warranty conditions for the handpiece can be found in the operating manual for the corresponding handpiece.

Need help?
Do you have a question about the MASTERPULS MP100 and is the answer not in the manual?
Questions and answers