Table of Contents
Advertisement
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Abbott Eterna 16000
- Page 1 Charging System Eterna™ Spinal Cord Stimulation System Model 16000 User's Guide...
- Page 2 State of California to cause cancer and birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov. ™ Indicates a trademark of the Abbott group of companies. ‡ Indicates a third-party trademark, which is property of its respective owner.
-
Page 3: Table Of Contents
Contents About This Guide..............1 Prescription and Safety Information. -
Page 5: About This Guide
This charger is not serviceable by the customer. To prevent injury to yourself or damage to the charging system, do not modify the charger. If needed, return the charger to Abbott Medical for service. Battery warning. The charger contains a lithium‑ion battery and other potentially hazardous materials. -
Page 6: Precautions
Use of accessories, transducers, and cables other than those specified or provided by Abbott Medical could result in increased electromagnetic emissions or decreased electromagnetic immunity and result in improper operation of the system. -
Page 7: Adverse Effects
the charging system. To correct the effects of typical system interference, keep the charging system at least 0.5 m (1.6 ft) from these types of wireless communication equipment. If you continue to have problems with interference, further increase the distance between the equipment and your charging system. Charge your charger. - Page 8 Power adapter The power adapter connects with an electrical outlet and with the charging cable to charge the charger battery. Charging cable The charging cable connects with the power adapter and with the charger to charge the charger battery. Charging apparel –...
-
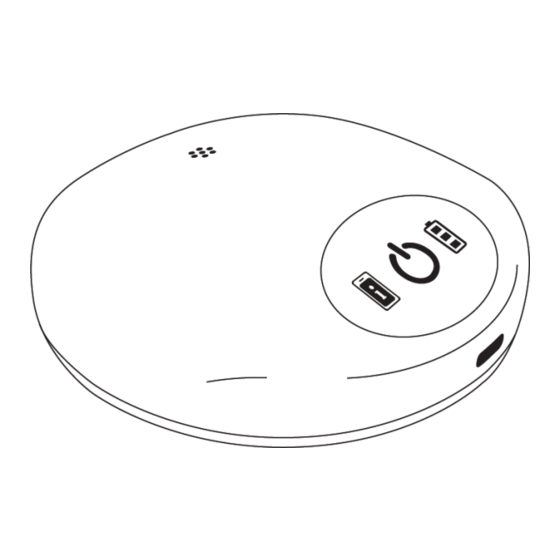
Page 9: Charger Indicators
Charger Indicators The charger has a power icon, a battery icon, and a patient controller alert icon as shown in the figure below. The charger provides indicator lights and sounds that show the charger status and the action being performed. ▪... -
Page 10: Using Your Charger
Using Your Charger The instructions in this section explain how to use your charging system to charge your generator battery and charger battery, how to use the charging apparel, and how to care for and travel with your charging system. NOTE: Each time before using the system, inspect the device and its accessories for damage. -
Page 11: Charging Your Charger
▪ ▪ When stimulation has stopped due to generator battery depletion Your patient controller indicates the battery level of your generator. The level ranges from 0% charge to 100% charge. The patient controller also indicates the approximate stimulation time remaining based on the generator’s current battery level and therapy settings. -
Page 12: Setting Up The Charging Apparel
To charge your charger, follow these steps: 1. If the charger is in the charging apparel, remove the charger from the apparel before charging. NOTE: The charging apparel is not intended to be used while charging the charger. 2. Plug the power adapter into an electrical outlet. NOTE: While the charger is charging, it should be placed on a stable surface, such as a table, counter, or floor. - Page 13 patients with generators implanted in the lower back may choose to use the lumbar charging apparel like a belt. If you choose to use your charging apparel, you should adjust it for comfort and fit before charging your generator. Your clinician will help you find the configuration that is most comfortable and suitable for your generator charging sessions.
- Page 14 4. Ensure the elastic edges of the pocket overlap, fully covering the charger. NOTE: ▪ When the generator enters the charging zone, it beeps continuously until ▪ charging begins. ▪ If the charger does not find the generator within 90 seconds, the charger beeps ▪...
- Page 15 Double-strap configurations Option 1 – Loop and charger on opposite sides 1. Release the counterweight from the charger pocket. 2. Grasp the plastic buckle so the charger pocket is hanging toward your feet. NOTE: If your generator is located on the right side of your body, grasp the buckle with your right hand.
-
Page 16: Charging Your Generator
6. Fasten the buckle. NOTE: If needed, use the excess strap near the buckle to tighten or loosen the apparel. Charging Your Generator After you have charged your charger and have set up the charging apparel (if desired), you are ready to charge your generator. It is recommended to charge your generator frequently and to charge before stimulation turns off due to a low battery. -
Page 17: Caring For And Maintaining Your Charger And Charging Apparel
4. To confirm charging is occurring, note the following. If you do not note any of the indicators listed below, charging is not occurring. – If the charger sound is enabled in the charger settings on the patient controller, – –... -
Page 18: Traveling With Your Charging System
Table 2. Storage, transportation, and operating conditions Temperature excursions Excursions below 15°C (59°F) or above 30°C (86°F) should be <24 hours in duration Temperature excursion -20°C (-4°F) to 60°C (140°F) limits Humidity 5% to 90% RH Pressure 70 kPa to 106 kPa Operating conditions Temperature 5°C (41°F) to 35°C (95°F) -
Page 19: Troubleshooting Charging Issues
▪ Refer to the terms and conditions for repair or replacement of Abbott Medical ▪ neurostimulation system components as stated in the Limited Warranty card included in your product documentation. Troubleshooting Charging Issues The following table lists possible charging system problems and possible solutions. - Page 20 Table 3. Troubleshooting charging issues Problem Possible Solution Reposition the charger, aligning the bottom of the charger with the bottom edge of the generator. Ensure the charging apparel is centered over the generator. Tighten the charging apparel to ensure the charger is positioned correctly over the generator.
-
Page 21: Service And Ordering Information
Technical Support For technical questions and support for your product, use the following information: ▪ ▪ +1 855 478 5833 (toll-free within North America) ▪ ▪ +1 651 756 5833 For additional assistance, call your local Abbott Medical representative. -
Page 22: Appendix A: Regulatory Statements
Ordering Information To order parts, contact Technical Support. Refer to the following list for order numbers. Table 4. Ordering information for the charging system Description Model Charger kit – lumbar 36000 Charger 16000 Charging apparel - lumbar 16750 Charging apparel - pectoral 16760 Travel case 16730... -
Page 23: Product Classification Statement (Cispr 11, Class B)
Operation is subject to the following two conditions: ▪ ▪ This device may not cause harmful interference. ▪ ▪ This device must accept any interference received, including interference that may cause undesired operation. Modifications not expressly approved by the manufacturer could void the user’s authority to operate the equipment under FCC rules. -
Page 24: Quality Of Service For Wireless Technology
Table 7. Bluetooth® Low Energy (LE) wireless technology information Channel 40 logical channels using AFH** Bandwidth per channel 2 MHz Data flow Bi-directional Protocol Bluetooth LE wireless technology Semi-duplex capability *EIRP = Equivalent isotropically radiated power (not duty cycle adjusted) **AFH = Adaptive frequency hopping The radio receiver in the device is using the same frequency and bandwidth as the transmitter. -
Page 25: Wireless Security Measures
Wireless Security Measures The wireless signals are secured through device system design that includes the following: ▪ ▪ The charger encrypts its wireless communication with the programming device using a key that is unique to that link. ▪ ▪ Only one patient controller or clinician programmer may communicate with the charger at a time. -
Page 26: Appendix C: Technical Specifications
Minimum 20 V Current rating Up to 3 A Cable length USB‑IF Certified Only use an Abbott Medical charging cable (model 16710) or a cable marked with USB-IF certification such as the following: Power adapter ratings Power 5.25 W maximum Input voltage 100‑240 VAC... -
Page 27: Appendix D: Electromagnetic Compatibility Guidelines
To avoid increasing emissions or decreasing immunity from a device or system, ▪ ▪ ▪ use only components approved by Abbott Medical with this system. Do not use Abbott Medical components with devices or systems that are not approved by Abbott Medical. Table 11. Guidance and Manufacturer's Declaration - Electromagnetic Emissions... - Page 28 Table 11. Guidance and Manufacturer's Declaration - Electromagnetic Emissions Emissions Test Compliance Electromagnetic Environment Guidance power supply network that supplies Voltage fluctuations/ flicker emissions Class 5 Full Compliance* buildings used for domestic purposes. IEC 61000-3-3 *Only applicable to Power Adapter (Model 16720) Table 12.
- Page 29 WARNING: Do not use portable RF communications equipment (including peripherals such as antenna cables and external antennas) closer than 30 cm (12 inches) to any part of the device, including cables specified by Abbott Medical. Otherwise, performance degradation may occur.
-
Page 30: Appendix E: Symbols And Definitions
Pulse modulation 50 kHz Appendix E: Symbols and Definitions The symbols below and harmonized symbols may be found on the product or product label. For harmonized symbols, refer to the Universal Symbols Glossary at medical.abbott/manuals. Table 16. Symbols and definitions Symbol Definition... - Page 31 Table 16. Symbols and definitions Symbol Definition NOTE: Magnetic Resonance (MR) Unsafe, an item poses unacceptable risks to the patient, medical staff, or other persons within an MR environment. Non-ionizing electromagnetic radiation Use-by date Class II Equipment Keep dry; keep away from rain Ingress protection rating for a device that is protected from the intrusion of solid foreign objects as small as 12.5 mm in diameter and is protected from vertically dripping water when the device is...
- Page 32 Authorized representative in the European Community European conformity, affixed according to the relevant provisions of European Council Regulation 2017/745 (NB 2797) and RE directive 2014/53/EU Annex II. Hereby, Abbott Medical declares that this device complies with the relevant provisions of this regulation and directive.
- Page 33 Table 16. Symbols and definitions Symbol Definition This device is a recognized component by Underwriters Laboratories (UL) Inc. This device complies with part 18 of the FCC rules.
- Page 36 2023-05 ARTEN600283824 B *600283824*...
Need help?
Do you have a question about the Eterna 16000 and is the answer not in the manual?
Questions and answers
Why the charger is blinking green after unplugging it