Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Avita BPM19
- Page 1 Wrist Type Blood Pressure Monitor BPM19 RoHS REACH MDSS GmbH Schiffgraben 41 30175 Hannover Germany AViTA Corporation 9F., No. 78, Sec. 1, Kwang Fu Rd., San Chung Dist., New Taipei City 24158, Taiwan. China, AViTA(WUJIANG) Importer Distributor...
-
Page 2: Table Of Contents
INSTRUCTION MANUAL Please read this instruction manual carefully before operating this unit Contents Intended Use........................1 Important Information Before Use ................5 Product Identification ..................... 8 Description of LCD Display ................... 9 Battery Installation ......................11 Setting the Date and Time ..................13 Applying Your Cuff ...................... - Page 3 Cleaning and Disinfecting ................... 29 Technical Specification ....................30 EMC Tables ......................... 32 Explanation of Symbols ....................36...
-
Page 4: Intended Use
Intended Use The product automatically measures human begins Systolic, Diastolic blood pressure and pulse rate by oscillometric method. The measurement results are displayed on the LCD. Measurement position is at human being's arm. The intended use of this over-the-counter device is for adults with arm circumference ranging from 125 mm to 210 mm (Approx. - Page 5 CAUTION: • Beware of blood flow interference and resulting harmful injury to the patient caused by continuous cuff pressure. • Pressurization of the cuff can temporarily cause loss of function of monitoring ME equipment simultaneously being used on the same limb.
- Page 6 • Ensure that the cuff is not placed on an arm in which the arteries or veins are undergoing medical treatment, e.g., intravascular access or therapy, or an arteriovenous (AV) shunt. • This product is suitable for use in the home healthcare environment. •...
- Page 7 • Cuff pressure 0 – 300 mmHg • Reduction rate: ≦30S Refer to IEC 80601-2-30 • No modification of this equipment is allowed.
-
Page 8: Important Information Before Use
Important Information Before Use Blood pressure measurements should only be interpreted by a physician or a trained health care professional who is familiar with your medical history. Through regular use of this device and recording of your measurements, you can keep your physician informed of the changes in your blood pressure. - Page 9 accurate reading. Remain still; do not talk during the measurement. Record your daily blood pressure and pulse readings on a chart. Take your readings at the same time, each day or as recommended by your physician to get an accurate indication of change in your true blood pressure.
- Page 10 For Customer Service, It is recommended that the accuracy should be checked by manufacture every 2 years. To obtain the service please contact AViTA Corp. for the address of the repair location. Enclose the Proof of Purchase. Include $10.00 USD for the return shipping and handling.
-
Page 11: Product Identification
Product Identification... -
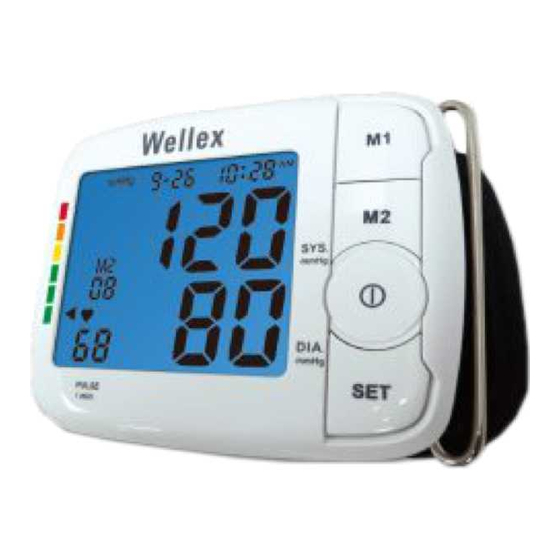
Page 12: Description Of Lcd Display
Description of LCD Display Memory sets Memory Symbol Average Measurement Symbol Low Battery Symbol Inflation Symbol Deflation Symbol Date... - Page 13 Time Digital Display Pulse Rate Heartbeat Symbol Hypertension Indicator Irregular Heart Beat Symbol...
-
Page 14: Battery Installation
Battery Installation Low battery warning: It is necessary to replace the batteries when the Low Battery symbol appears on the display, or when the display does not turn on after the POWER button is pressed. Replacing the Battery: - Open the battery cover on the bottom of the monitor. - Insert or replace 2 x 1.5 V AAA batteries into the battery compartment, ensuring to match the indicated polarity symbols. - Page 15 greatly in quality and strength. Use of rechargeable batteries may affect the performance of this device. - Please properly dispose of the batteries away from small children and heat. - It is recommended to remove the batteries if the unit will not be used for an extended period of time.
-
Page 16: Setting The Date And Time
Setting the Date and Time It is recommended to set the date and time for the unit every time batteries are initially installed or replaced. When the unit is off (a blank display screen) or after replacing batteries, press and release the “Date and Time” button; the “Year”... -
Page 17: Applying Your Cuff
Applying Your Cuff It is important to avoid smoking, eating, taking medication, alcohol consumption or physical activity 30 minutes prior to taking a reading. If for any reason you are unable to or should not use your left wrist, please modify the instructions for cuff application to your right wrist. Your physician can help you identify which wrist is best for you to take measurements from. - Page 18 of your wrist facing you. - Adjust the cuff approx. 1 cm from the edge of the head of the ulna bone. - The cuff should fit comfortably, yet snugly around your wrist. - Blood pressure naturally varies from one wrist to the other;...
-
Page 19: Measurement Of Pulse Rate And Blood Pressure
Measurement of Pulse Rate and Blood Pressure Please read the preceding portions of this manual prior to taking your first reading. Rest your elbow on a solid surface with your palm facing upward. Elevate your wrist so that the cuff is at the same level as your heart. - Page 20 When the inflation has reached optimum level, the display will begin to show the decreasing pressure; the screen will display an arrow pointing down while you feel the pressure of the cuff decrease. To detect the heartbeat, the heartbeat symbol will appear and continuous flashes on the LCD display.
-
Page 21: Hypertension Indicator
Hypertension Indicator This monitor comes equipped with a Hypertension Indicator that automatically compares each reading to defined levels established by the World Health Organization and provides a helpful cue if your reading falls into one of the stages that could potentially indicate increased risk. -
Page 23: Irregular Heartbeat Detector
Irregular Heartbeat Detector Your digital blood pressure monitor features an Irregular Heartbeat Detector. Irregular Heartbeats may influence the results of the measurement. If the monitor detects the Irregular Heartbeats during measurement, the symbol will appear on the display with the measurement values. - Page 24 IMPORTANT INFORMATION: This blood pressure monitor is not designed for use by people with arrhythmias nor for diagnosing or treating an arrhythmia problem. As a safeguard, we recommend that if you have arrhythmias such as atrial or ventricular premature beats and atrial fibrillation or any other special conditions you should check with your physician before using your blood pressure monitor.
-
Page 25: Memory Function
Memory Function Recalling Measurements in Memory: Press and release the “MEMORY” button. The unit will first display the average of the last 3 stored measurements. Continue to press the “MEMORY” button to successively view the next previously stored measurements. Measurements will appear on the display from most current to oldest;... - Page 26 Clearing Measurements from Memory: From power display off, press and hold down the “MEMORY” button until the display shows CLr. This indicates that all measurements have been erased.
-
Page 27: Error Codes
Error Codes Error Cause Corrective Action Code Err 00 No pulse or detect pulses Take off heavy clothes and retry again. not enough. Err 01 Leakage in Cuff The wrist cuff is not fastened properly. Pressure/Inflation too low. Reapply the cuff, and take a measurement again. - Page 28 Error Cause Corrective Action Code Low batteries Replace all batteries with new ones.
-
Page 29: Troubleshooting
Troubleshooting Problem Cause Remedy The reading The wrist cuff is not at Measure while in the correct is extremely heart level. posture. low (or The cuff is not wrapped Wrap the cuff correctly. high). snugly around the wrist. The arms and Relax and try taking the shoulders are tense. - Page 30 Problem Cause Remedy The blood pressure is different each Blood pressure readings time. The reading is extremely low (or constantly vary with time of high). day and how relaxed you are. Take several deep breaths and try to remain relaxed before taking a measurement.
- Page 31 Problem Cause Remedy Errors with The machine must be wrist positional adjusted according to the instruction manual and with professional...
-
Page 32: Cleaning And Disinfecting
Cleaning and Disinfecting • Clean your blood pressure monitor carefully using a slightly damp cloth only. • Do not use any detergents or solvents. • Never hold the instrument under water as otherwise liquid can penetrate and damage the instrument •... -
Page 33: Technical Specification
Technical Specification • Measuring range : Blood Pressure : 30~280 mmHg Pulse Rate : 40~199 beats/min • Calibration Accuracy : Pressure : ± 3 mmHg Pulse rate : ± 4% of reading • Operating environment : 10℃~40℃ (50℉~104℉) with relative humidity up to 85% (non condensing) Atmospheric Pressure: 700~1060 hPa •... - Page 34 • Weight : approx. 90g (exclude batteries) • Dimensions : approx. 91mm × 74.5mm × 15mm (L×W×D) • Cuff circumference : approx. 12.5~21 cm • Life time: 3 years; • Battery life: above 300 times...
-
Page 35: Emc Tables
EMC Tables BPM19 is intended for use in the electromagnetic environment specified below. The customer or the user of BPM19 must make sure that it is used in such an environment. Guidance and manufacturer’s declaration - Electromagnetic emissions CISPR 11... - Page 36 Group 2 Class A (With BLE Function) a) The equipment is suitable for use in Home Health Environments and Professional Health Care Environments limited to patient rooms and respiratory treatment facilities in hospital or clinics. The more restrictive acceptance limits of Group 1 Class B (CISPR 11) have been considered and applied.
- Page 37 RF communications equipment (transmitters) and the BPM19 can be requested from supplier using the contact information provided in this manual. However, it is advisable to keep the electromechanical aerosol equipment at an adequate distance of, at least, 0.5 m from mobile phones...
- Page 38 frequency 50 Hz or 60 Hz magnetic fields. a) The equipment is suitable for use in Home Health Environments and Professional Health Care Environments limited to patient rooms and respiratory treatment facilities in hospital or clinics. The more restrictive IMMUNITY acceptance limits have been considered and applied. b) Before modulation is applied.
-
Page 39: Explanation Of Symbols
Explanation of Symbols The CE marking with the Registration Number of the Notified Body. This denotes the compliance of Regulation (EU) 2017/745 Medical Device Manufacturer... - Page 40 Authorized representative in the European Community Date of manufacture (YYYY-MM-DD or YYYY-MM) Batch code (YYMMWWWW) Serial number (YYMWWWXXXXX)
- Page 41 Keep dry Temperature limit Humidity limitation Atmospheric pressure limitation...
- Page 42 Caution Consult the instruction for use Disposal information: Should you wish to dispose of the article, do so in accordance with current regulations. Details are available from your local authority. WEEE 2012/19/EU Directives This product fulfilling the requirements of the RoHS RoHS Directive 2011/65/EU.
- Page 43 This product fulfilling the requirements of the REACH Directive EC 1907/2006 and its amendments, do not contain Substances of Very High Concern in REACH concentration above the limit of 0.1 %. No substance(s) is/are present in the parts of the product above the concentration of 0.1 % weight by weight.
- Page 44 This product meets the basic safety and essential performance requirements indicated in the IP22 conditioning test (protection against solid foreign objects of 12.5mm ∅ and greater and against vertically falling water drops when enclosure tiled up to 15°) The empty, completely flat batteries must be disposed of through specially designated collection boxes, recycling points or electronics retailers.
- Page 45 Distributor Model Number Country of Manufacturer Unique Device Identifier BPM19P-22001AV 2022-12-14...












Need help?
Do you have a question about the BPM19 and is the answer not in the manual?
Questions and answers